Understanding Ribosome Structure: Types, Diagrams & Key Roles
Imagine a bustling factory working around the clock—this is exactly what ribosomes do inside every living cell. Acting as tiny production units, these complex organelles read genetic instructions and build proteins that power life. Whether you’re a student curious about what is ribosomes or a parent exploring about ribosomes to help your child understand biology, this page provides a complete guide covering their definition, structure, function, and even the types of ribosomes found in nature.
What are Ribosomes?
Ribosomes Definition:
Ribosomes are essential cell organelles made up of RNA and proteins. They are often described as the cell’s protein factories because they translate the genetic code from messenger RNA (mRNA) into amino acid chains— the building blocks of proteins. Every living cell, whether prokaryotic or eukaryotic, contains ribosomes to perform this crucial function.
Structure of Ribosomes
Understanding the structure of ribosomes is key to grasping how they work. Ribosomes are ribonucleoprotein complexes consisting of two subunits:
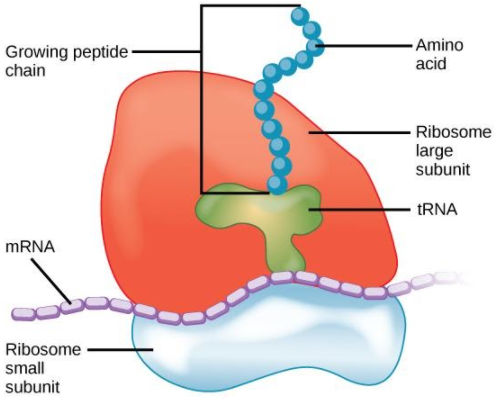
Small Subunit: Binds to mRNA and decodes its genetic message.
Large Subunit: Joins amino acids together, forming the protein chain.
In prokaryotes, ribosomes are known as 70S ribosomes, while eukaryotes possess 80S ribosomes. A ribosomes diagram would typically illustrate these subunits, highlighting that approximately 62% of their mass is RNA and the rest is protein. Both free and bound ribosomes perform protein synthesis, though the latter is often involved in exporting proteins outside the cell.
Function of Ribosomes
The function of ribosomes centres on protein synthesis, an essential process for life:
Transcription: DNA is transcribed into mRNA in the nucleus (in eukaryotes).
Translation: Ribosomes read the mRNA and use transfer RNA (tRNA) to add the appropriate amino acids.
Protein Assembly: The growing amino acid chain folds into a functional protein.
Proteins produced by free ribosomes usually remain within the cell, while those made by bound ribosomes are destined for secretion or membrane integration.
Types of Ribosomes
There are two main types of ribosomes based on their location and function:
Free Ribosomes: Floating in the cytosol, synthesising proteins that function within the cell.
Bound Ribosomes: Attached to the endoplasmic reticulum (ER), producing proteins for export or membrane integration.
Interactive Quiz & Fun Task
Test your understanding of ribosomes with this quick quiz and engaging activity!
Quiz: Check Your Answers
What is the primary role of ribosomes?
Answer: They synthesize proteins by translating mRNA.
Which ribosome subunit binds to mRNA?
Answer: The small subunit.
Name the two types of ribosomes based on their location.
Answer: Free ribosomes and bound ribosomes.
What percentage of a ribosome is typically made up of RNA?
Answer: Approximately 62%.
How do ribosomes contribute to the process of protein synthesis?
Answer: They translate genetic code into amino acid sequences.
Fun Facts about Ribosomes
Tiny Titans: Despite their microscopic size, ribosomes are present in millions per cell!
Ancient Origins: Ribosomes are among the most evolutionarily conserved components in all living organisms.
Dual Presence: They exist both freely in the cytosol and attached to the endoplasmic reticulum, each serving distinct roles.
Real-World Applications
Ribosomes play a pivotal role in biotechnology and medicine. For instance:
Drug Development: Many antibiotics target bacterial ribosomes to inhibit protein synthesis without affecting human cells.
Genetic Engineering: Understanding ribosome function aids in designing synthetic biology applications to produce therapeutic proteins.
Disease Research: Defects in ribosome production or function can lead to ribosomopathies, a group of disorders with significant health impacts.


FAQs on Ribosomes: Structure, Function, and How They Make Proteins
1. What are ribosomes and what is their primary role in a cell?
Ribosomes are complex molecular machines found within all living cells that serve as the primary site for protein synthesis. Composed of ribosomal RNA (rRNA) and proteins, their main function is to read the genetic instructions from messenger RNA (mRNA) and translate that code into a specific sequence of amino acids, creating a polypeptide chain that folds into a functional protein.
2. Why are ribosomes often called the 'protein factories' of the cell?
Ribosomes are called the 'protein factories' because they are the central workbench where all proteins are manufactured. Just like a factory takes raw materials (amino acids) and follows a blueprint (mRNA) to create a finished product (protein), ribosomes assemble proteins that are essential for virtually all cellular activities, including enzyme function, structural support, and transport.
3. What is the fundamental structure of a ribosome?
Every ribosome consists of two distinct subunits: a smaller subunit that binds to the mRNA and a larger subunit that joins the amino acids to form the protein chain. These subunits are made of specific rRNA molecules and a variety of proteins. The two subunits only come together to form a functional ribosome when protein synthesis is initiated.
4. What is the key difference between the 70S and 80S ribosomes?
The primary difference lies in their size and where they are found. The 'S' stands for Svedberg unit, a measure of sedimentation rate during centrifugation.
- 70S ribosomes: These are smaller and found in prokaryotic cells (like bacteria) and also within the mitochondria and chloroplasts of eukaryotic cells. They consist of 50S and 30S subunits.
- 80S ribosomes: These are larger and are found in the cytoplasm and on the endoplasmic reticulum of eukaryotic cells (like in plants and animals). They consist of 60S and 40S subunits.
5. How do free and bound ribosomes differ in their function within a eukaryotic cell?
While structurally identical, their location determines the destination of the proteins they synthesise.
- Free ribosomes float in the cytoplasm and typically produce proteins that will be used within the cell itself, such as enzymes for metabolic pathways.
- Bound ribosomes are attached to the outer surface of the endoplasmic reticulum. They synthesise proteins destined for export out of the cell, insertion into cell membranes, or delivery to specific organelles like lysosomes.
6. How do ribosomes facilitate the process of translation?
Ribosomes facilitate translation by providing a framework with three key sites: the A (aminoacyl) site, the P (peptidyl) site, and the E (exit) site. The small subunit first binds to the mRNA. Then, transfer RNA (tRNA) molecules, each carrying a specific amino acid, enter the A site. The ribosome catalyses the formation of a peptide bond between the new amino acid and the growing polypeptide chain at the P site. The ribosome then moves along the mRNA, shifting the tRNAs to the next site, and the uncharged tRNA leaves from the E site.
7. If ribosomes make proteins, what makes the ribosomes?
This is a classic biology question. The 'factory' that produces ribosomes is the nucleolus, a dense structure found inside the nucleus of eukaryotic cells. The nucleolus is where ribosomal RNA (rRNA) is synthesised and combined with proteins (which are made by existing ribosomes in the cytoplasm and then imported into the nucleus) to form the ribosomal subunits. These subunits are then exported back to the cytoplasm to become functional ribosomes.
8. What is a polysome, and why is it an efficient mechanism for the cell?
A polysome, or polyribosome, is a structure formed when multiple ribosomes simultaneously translate the same mRNA molecule. It looks like 'beads on a string'. This mechanism is highly efficient because it allows the cell to produce a large number of copies of the same protein from a single mRNA transcript in a very short amount of time, meeting a high demand for that specific protein quickly.
9. How can some antibiotics target bacterial ribosomes without harming human ribosomes?
This is possible due to the structural difference between prokaryotic (70S) and eukaryotic (80S) ribosomes. Antibiotics like streptomycin and tetracycline are designed to specifically bind to and inhibit the function of the smaller 70S ribosomes found in bacteria. Because human cells have larger 80S ribosomes in their cytoplasm, these antibiotics do not affect our cellular protein synthesis, making them effective at killing bacteria while leaving the host cells unharmed.










