What is the Principle of pH Metry?
A pH indicator, also termed an acid-base indicator, is a halochromic chemical compound that is added in small amounts to a solution to determine the acidity or basicity of the solution visually. When added to a dilute chemical solution, the pH indicator does not affect the alkalinity or basicity of the solution.
pH indicators are weak acids or weak bases present as natural dyes and help indicate the hydrogen ion concentration in a solution through its colour change. The full form of pH is the Potential of Hydrogen, meaning the strength of hydrogen. It is the negative logarithm of hydrogen (H+) ion concentration. It is thus used to describe the concentration of H+ ions in a chemical solution specifying the acidic, basic, or neutral nature of the solution being tested.
Principle of pH Metry
A pH metre shows how much acidic or alkaline a chemical solution is. The basic principle of pH Metry is to measure the hydrogen ion concentration in the liquid that is being tested. The strength of the hydrogen ion, whether positive or negative, depends on two things.
When acids get dissolved in water, they form positively charged hydrogen ions (H+), whereas when bases or alkalis get dissolved in water, they form negatively charged hydrogen ions (OH-). The greater the concentration of H+ ions, the stronger the acid is.
Likewise, the higher the concentration of OH- ions in the solution, the stronger the base is. pH level is thus determined based on these hydrogen ions mixed solution dissolved in a certain amount of water.
A pH 7 colour indicates a neutral solution. This means that pure water must have a pH value of 7. Less than 7 implies an acidic solution, while greater than 7 implies an alkaline solution.
Digital pH Metre
Knowing about the pH Range of Indicators
It’s time to gather knowledge about the pH range of indicators. By now, we all know that pH indicators are used to visually identify the acidity or alkalinity of an aqueous, i.e., water-based solution. The acid-base pH indicators are chemical solutions that include a specific distinguishing colour at certain pH levels.
Every pH indicator usually changes colour for a range of two pH units. These indicators work only in colourless solutions such that the naked eye can easily observe any colour change and thus provide an approximate pH value.
Some widely used pH indicator examples include Phenolphthalein, Methyl Red, and Bromothymol Blue. These show pH ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5, respectively. The pH scale colours vary from one range to another:
Phenolphthalein goes from colourless to red. This means at low pH or in the acid form (8 and lower), the pH indicator solution is colourless, and at high pH or in the basic form (10 and above), it turns red. Between pH 8 and 10, the indicator turns pinkish.
Methyl Red ranges from red to yellow, meaning at low pH (4.5 and lower), the indicator solution is red, and at high pH (6 and above), the colour becomes yellow. The pH indicator solution is orange when the pH range is between 4.5 and 6.
Bromothymol Blue varies from yellow to blue, which means at low pH (6 and lower), the indicator displays yellow colour, and at high pH (7.5 and above), it turns blue. The indicator turns greenish when the pH value is between 6 and 7.5.
Universal Indicator pH Scale
A universal indicator pH scale is used to vary widely from 4 to 14 to determine the pH levels of various chemical solutions and substances. A universal indicator is a mixture of dyes that helps change the solution's colour.
The primary components in preparing this mixture are Thymol blue, Methyl red, Boromothymol blue, and Phenolphthalein. It is important to build this mixture very carefully as each ingredient will either lose or gain electrons based on the acidity or basicity of the chemical solution being tested.
It has been noticed that when this type of universal indicator is used in a colourless solution, the resultant solution obtained is as per the expectations. It also helps in increasing the accuracy of indications.
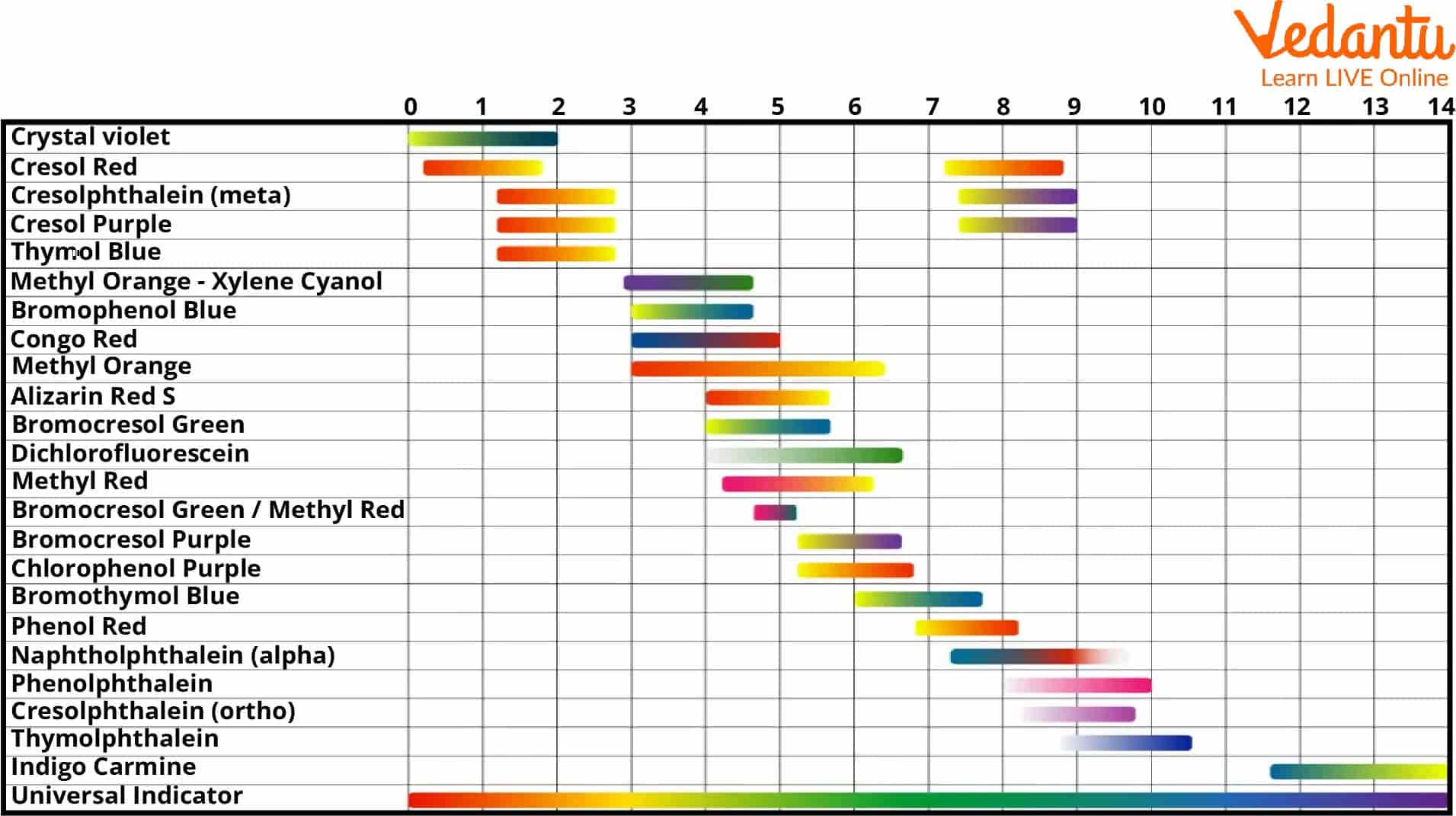
Universal Indicators
Uses of Universal Indicator pH Scale
This indicator exhibits acidity or alkalinity of diverse substances like sludge, soil, food, sewage, etc., solutions and liquids such as normal water, wastewater, etc.
Advantages of Universal Indicators
A universal indicator helps display a wide range of colours over the whole pH scale, thus making it possible to specify an approximate pH of a solution or substance over a range starting from 4 to 14.
Limitations of Universal Indicators
Before using a universal indicator, it is important to learn about the limitations.
It is not usually used in titration because it gradually changes, exhibiting different colours for different pH levels. This makes it challenging to determine the actual pH of the tested solution.
The resultant pH colour may be incorrect if a contaminated solution or the substance.
Applications of pH
Industries that use water as one of the main components would be required to test the pH levels periodically to ensure that water composition falls within the expected ranges. It is important to understand the function of pH in your daily life. Below are some of the common applications of pH in various industries:
Wastewater Treatment
Determining the pH level is crucial in the wastewater treatment method. Heavy metals, particulate matter, toxic chemicals, etc. are removed from the water during this process. The pH levels must be adjusted by adding certain chemicals to the water to separate these dissolved wastes from the liquid.
Food and Beverage
Industries producing food or beverage need to monitor those products' pH levels strictly. Lower pH levels in food indicate that it is not fresh and healthy to eat. Also, if the water used in beverage production is too acidic, it might cause severe damage to the consumers’ dentition. It must be kept in mind that consumers should restrict from having food or any drink that might be unsafe for their health.
Spa and Pool Water
To maintain a proper safe swimming pool or a spa environment, you need to measure the pH levels of water to specify the amount of sterilising agents used. Too high pH levels can cloud the water, irritating the swimmers’ eyes and skin. Again, too low pH levels can not only irritate the swimmers’ eyes and skin and damage the pool’s plaster.
Research Experiments
Water is an integral part of most research experiments. Hence, researchers need to measure the pH levels to ensure high-quality results that can be replicated.
Conclusion
Determining proper pH levels indicates how acidic or basic a solution or a substance is. It plays an important role in industrial and daily human life. A pH metre is used to measure precise pH levels.







