




An Overview of Class 11 Chemistry Determination Of Melting Point Experiment
The melting process is the most common and well-known physical change observed by one. The melting of a solid is an example of melting ice into water, melting butter in a pan, etc. These all show a state change from solid to liquid, and this change is known as melting.
The process of melting helps in the determination of a substance's melting point. Determination of the melting point of an organic compound is greatly useful to check the compound's properties, such as its bulky nature, nature of bonding, and force of attraction between molecules.
Table of contents
Aim
Apparatus Required
Theory
Procedure
The procedure of melting point determination of the given sample/compounds.
Table to identify the given sample/compound with known melting points
Determination of Boiling point of Organic Compounds
Observation
Result
Precautions
Aim
To determine the melting point of the given solid compounds and identify the nature of the compounds.
Materials Required
Iron stand
Thermometer
Tripod Stand
Beaker
Clamp
Kerosene burner
Capillary tube (9 to 10 cm long and 0.5 to 2 mm diameter)
Spatula
Paraffin oils
Theory
The melting point theory is greatly influenced by the nature of bonding and the force of attraction between molecules.
The following types of bonding present in the molecules are intermolecular forces, Van der Waal forces, dipole-dipole interactions, ion-ion interactions, and hydrogen bonding.
The bonding strength is as follows- Ion-ion interactions > Hydrogen bonding > Dipole-dipole interactions > London dispersion forces > Van der Waal forces.
Therefore, the force of attraction between molecules majorly affects the melting point of a compound. The stronger the force of attraction between molecules, the more the melting point of a compound/sample.
Carbon-bonded or non-polar compounds have weak Van der Waal forces of attraction and dipole-dipole interactions. Hence, the melting point for such compounds is generally low compared to polar compounds. Examples include phenol, oxalic acid, naphthalene, urea, etc.
The polar compounds have ion-ion interactions because of the electrostatic force of attraction and have very high melting points. Examples include sodium chloride, magnesium oxide, etc.
If the given samples show a drastic melting point difference between them, it shows the nature of bonding in the molecules.
A sharp melting point indicates the purity of the organic compound. It melts over a narrow range of temperature differences of about 0.5 °C to 1 °C. The organic compound contains impurities if the temperature difference exceeds this range.
Procedure
Take the given samples (1 and 2) and crush them into fine powder.
Take the capillary tube and prepare it by closing one end by heating it.
Put the capillary tube into the compounds at the open end.
The filling of the compounds is about 1-2 cm in the capillary tube by gently tapping it.
Attach the capillary tube with the thermometer with the help of a thread and attach it with a clamp.
Place the wire gauge on the tripod stand and keep a beaker containing paraffin oil.
Provide continuous heat to the beaker with a burner and dip the thermometer into it.
The melting point apparatus diagram is as follows-
The iron stand and clamp are set up initially, and the thermometer is attached to the clamp and inserted into the beaker.
The sample in the capillary tube is attached to the thermometer, which is inserted into the beaker.
The beaker filled with liquid paraffin is placed over the wire gauge, which is settled up by the tripod stand.
The continuous heat is supplied to the beaker to maintain the constant temperature by the Bunsen burner.
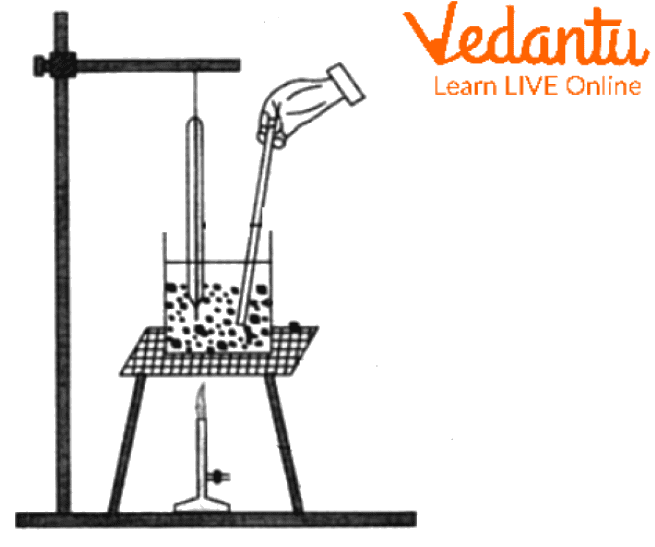
Melting Point Apparatus
Note the temperature when the given compounds/samples start melting and completely melt in the following manner-
The temperature when the compound starts melting, say the initial temperature (t1°C).
The temperature when the compound completely melts, says the final temperature (t2°C).
The correct melting point of the compound is determined by an average of two temperatures, t1, and t2, and the formula is - \[\left( {\dfrac{{t_1^^\circ C + t_2^^\circ C}}{2}} \right)\].
Observation
Melting Point of Sample 1
Melting Point of Sample 2
Identification of given samples (observed melting points) with known melting points of organic compounds
From the above data, the melting point of sample 1 is 79.60 °C, which is similar to naphthalene.
The melting point of sample 2 is 122.2 °C, which is similar to benzoic acid.
Result
From the above analysis, sample 1 is naphthalene and sample 2 is benzoic acid.
The different melting points of the two samples are due to differences in their structure and nature of bonding.
The compound Naphthalene (C10H8) has a fused or condensed ring of hydrocarbon compound composed of two benzene rings sharing two adjacent carbon atoms. It has two rings with hydrogen bonding that make it a weak compound due to Van der Waals forces and non-polarity.
Whereas, the compound, benzoic acid (C6H5COOH), is composed of only one benzene ring with a carboxyl group. But it has intermolecular forces consisting of hydrogen bonding, dipole-dipole forces, and London dispersion forces.
Therefore, benzoic acid has a higher melting point when compared to naphthalene.
The above experiment for the determination of the melting point of a compound gives the nature of bonding exhibits in the molecules. Hence, the melting point can be explained by one.
Precautions
Always use powdered and dry samples (organic compounds).
The packaging of the organic compound should be uniform in the capillary tube, with no air gaps between the solid particles.
It is a necessary thermometer and capillary tube at the same level.
Do not overfill the capillary tube, and gently tap the capillary tube after filling it with the sample.
Always supply uniform heating to the sample.
Lab Manual Questions
1. What is melting point?
Ans. The melting point is usually defined as the point at which materials changes from a solid to a liquid. The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid. This is the point at which both liquid and solid phase exists at equilibrium.
2. What liquid determines the melting point and why?
Ans. The liquid paraffin oil determines the melting point because it has a high boiling point and can maintain a high temperature without losing the substance.
3. What is the melting point range of the organic compound?
Ans. The range where the melting point of the organic compounds can be varied and accepted is about 1 °C to 2 °C.
4. Why should the melting point sample be finely powdered?
Ans. The organic sample should be finely powdered because it gives maximum surface area in contact with heat, and the heat is uniformly distributed and gives an exact melting point of the compound.
Viva Questions
1. Is the melting point qualitative or quantitative?
Ans. The melting point is the physical properties that are easy to measure. Therefore, it is a quantitative property.
2. What are the factors that influence the melting point?
Ans. The following factors affect the melting point are
Size of the molecule.
The shape of molecules.
Intermolecular forces.
Ionic bonds.
3. Do impurities lower the melting point?
Ans. The presence of impurities disrupts the crystalline lattice of the organic compound and weakens the intermolecular force of attraction. Hence, it lowers the melting point.
4. Which organic compound or group has the highest melting point?
Ans. The amide group has the highest melting point due to the formation of intermolecular hydrogen bonding.
5. Can two compounds have the same melting point?
Ans. In the presence of millions of organic compounds, most of the compounds have melting points below 250 °C. Therefore, two different chemical compounds can have identical melting points.
6. Why is it necessary to slowly heat your sample when taking a melting point?
Ans. It is necessary to heat the beaker containing liquid slowly to maintain the thermal equilibrium between the liquid and the thermometer. A slow change in the physical state of the compound and temperature on the thermometer is observed.
7. What does it mean if the melting point was lower than the literature value?
Ans. If the melting point is lower than the literature value, it is due to the presence of impurities.
8. Why is the melting point a physical property?
Ans. Physical property is a change in the physical state of the compound. When an organic compound is heated, its solid state changes into liquid, but the composition remains the same. Therefore, the melting point is a physical property.
9. Does the molar mass of an organic compound affect the melting point?
Ans. Yes, the greater the molar mass, the higher the melting point.
10. What factors increase the melting point?
Ans. All organic compounds have different compositions, the nature of bonding, and the force of attraction between molecules. Polarity, especially hydrogen bonding, leads to a high melting point. Therefore, the polar organic compound has higher melting points than non-polar compounds of similar sizes.
Practical Questions
1. What is the name of the process that occurs when a solid changes to a liquid?
Freezing
Melting
Heating
Boiling
Ans. Melting is the process that changes the solid state into liquid.
2. The melting point of the ice is
O degrees Fahrenheit
0 degrees Celsius
100 degrees Celsius
100 degrees Fahrenheit
Ans. 0 degree Celsius
3. You melt a compound, and it disappears: what should you do?
Compound changes to a liquid.
Compound sublimes.
Impurity is present in the compound.
A pure compound is present.
Ans. Compound sublimes.
4. What would affect the observed melting point if the sample was packed too much?
The melting point will be very high.
The melting point will be shallow.
The exact and sharp melting point is observed.
The sample will melt quickly, and a low melting point will be observed.
Ans. The sample will melt quickly, and a low melting point will be observed.
5. What would affect the observed melting point if the sample was heated too quickly?
The results are better.
The sample is pure and does not have impurities.
The reading at which the sample starts to melt, and the reading at which the sample is wholly melted, will not be able to be read properly.
There is no sample left in the capillary tube.
Ans. The reading at which the sample starts to melt, and the reading at which the sample is wholly melted, will not be able to be read properly.
6. What would affect the observed melting point if the sample was not completely dry?
The compound would likely give the temperature at which its water evaporates.
The melting point will be lowered because water is impure.
The melting point observed is higher.
The melting point is sharp and exact of the compound.
Ans. The melting point will be lowered because water is impure.
7. You think you have isolated Tylenol in the lab. Since you don't trust your laboratory techniques, you want to prove to yourself that you have Tylenol before you ingest it. Using only melting point techniques, explain how you can prove that you have Tylenol.
Prepared samples subjected to heat to observe the melting point.
Mix the substance you just isolated (presumably Tylenol) with actual Tylenol in a 1:1 ratio, and find the melting point of that mixture. Then compare that to the melting point of the actual Tylenol to determine if your isolated substance is Tylenol.
The compound is to be mixed with water to observe the melting point.
The impurities are removed from the sample to prepare the compound.
Ans. Mix the substance you just isolated (presumably Tylenol) with actual Tylenol in a 1:1 ratio, and find the melting point of that mixture. Then compare that to the melting point of the actual Tylenol to determine if your isolated substance is Tylenol.
8. Why is it not successful to mix two components by simply stirring and then sampling them for the melting point?
This results in a very high melting point.
This results in a shallow melting point.
The two components mixing and stirring do not give a homogenous mixture, as a small and mixed sample would not represent both components.
The sample results are impure.
Ans. The two components mixing and stirring do not give a homogenous mixture, as a small and mixed sample would not represent both components.
9. Which organic compound has the maximum melting point among the following?
Bromobenzene
o-dibromobenzene
p-dibromobenzene
M-dibromobenzene
Ans. p-dibromobenzene has the highest melting point because of the symmetry of the molecule.
10. Which of the following elements has the highest melting point?
Zinc
Manganese
Iron
Chromium
Ans. Chromium has the highest melting point and the tendency to form metallic bonding due to the presence of unpaired electrons in its d-shell.
Conclusion
From the above experiment, we concluded that the solid melting point depends on the force of attraction between molecules. The presence of hydrogen bonding and stronger intermolecular compounds have a higher melting point. Organic compounds with lower melting points have weak Van der Waal forces of attraction. Therefore, melting point is one of the physical properties of an organic compound. The melting point of compounds depicts the nature of bonding between atoms.
FAQs on Class 11 Chemistry Determination Of Melting Point Experiment
1. How is the melting point of a crystalline solid defined for a Class 11 exam?
For a Class 11 exam, the melting point of a crystalline solid is defined as the specific temperature at which the solid phase changes into its liquid phase at atmospheric pressure. At this point, the solid and liquid phases exist in equilibrium.
2. Why is determining the melting point of a compound considered an important experiment in chemistry?
Determining the melting point is crucial for two main reasons tested in the CBSE Class 11 curriculum for 2025-26:
- Purity Assessment: A pure crystalline substance has a sharp and characteristic melting point. The presence of impurities lowers the melting point and causes it to melt over a wide range of temperatures.
- Identification of Substances: It is a physical constant that can help in identifying an unknown substance by comparing its experimentally determined melting point with known literature values.
3. How do impurities typically affect the melting point of a pure organic compound?
Impurities disrupt the uniform crystalline lattice structure of a pure compound. This disruption weakens the intermolecular forces, meaning less thermal energy is required to break the bonds. Consequently, the presence of impurities almost always lowers the melting point and causes the substance to melt over a broader temperature range rather than at a single, sharp point.
4. What is the significance of obtaining a sharp melting point versus a broad melting range?
The nature of the melting point provides critical information about the sample:
- A sharp melting point (melting over a narrow range of 1-2°C) is a strong indicator of a substance's high purity.
- A broad melting range indicates that the substance is impure or is a mixture of different compounds. This distinction is a key concept frequently asked in practical exams.
5. What are the key precautions a student must take when performing the melting point determination experiment for the CBSE Class 11 practicals (2025-26)?
For accurate results in the CBSE Class 11 practical exam, students should follow these precautions:
- The capillary tube must be packed with a finely powdered and completely dry sample.
- The heating of the liquid bath should be slow and uniform, especially when approaching the expected melting point.
- The thermometer and the capillary tube should be at the same level, with the substance positioned close to the middle of the thermometer bulb.
- Stir the liquid in the bath continuously to ensure uniform temperature distribution.
6. In the laboratory setup, why is paraffin oil or concentrated sulphuric acid used in the heating bath instead of water?
Paraffin oil or concentrated H₂SO₄ is used because they have a high boiling point and are stable at high temperatures. Most organic compounds have melting points well above the boiling point of water (100°C). Using water would limit the experiment to substances that melt below 100°C, and the water would evaporate quickly, making it unsuitable for a reliable heating bath.
7. Why is it crucial to heat the apparatus slowly and uniformly when approaching the melting point?
Heating slowly and uniformly is vital to ensure that heat is transferred equally to the substance, the liquid bath, and the thermometer at the same rate. If heated too quickly, the thermometer reading will lag behind the actual temperature of the bath and the sample. This leads to an inaccurate and erroneously high observed melting point, a common error in practical exams.
8. Explain the role of latent heat of fusion during the melting process. Why does the temperature remain constant?
The temperature of a substance remains constant at its melting point, even though heat is continuously supplied. This is because the added heat energy, known as the latent heat of fusion, is used to overcome the intermolecular forces holding the particles in the fixed crystal lattice. It is converted into the potential energy of the molecules to facilitate the state change, rather than increasing their kinetic energy, which would raise the temperature.
9. How can the melting point of a substance be used to identify an unknown organic compound?
To identify an unknown compound, its melting point is first determined experimentally. This value is then compared with the known melting points of standard compounds. A more definitive test is the mixed melting point determination. The unknown substance is mixed with a small amount of a known pure compound. If the mixture melts at the same sharp temperature as the pure compound, the unknown is identified. If it melts at a lower, broader range, it is a different substance.
10. What are some common sources of error that can lead to an inaccurate melting point determination in a school laboratory?
Several factors can lead to errors in this experiment, which are important to know for viva-voce and theory questions:
- Rapid Heating: Causes the thermometer reading to lag, resulting in an observed melting point that is higher than the actual value.
- Poor Packing of Sample: Loose packing in the capillary tube can lead to inefficient heat transfer and a broad, inaccurate melting range.
- Using a Wet Sample: The presence of a solvent like water acts as an impurity and will lower the melting point.
- Parallax Error: Incorrectly reading the thermometer scale from an angle.
























