




An Overview of Class 11 Chemistry To Prepare Crystals Of Pure Copper Sulphate From A Given Impure Sample Of The Blue Vitriol Experiment
By applying the recrystallisation method, it is possible to create pure copper sulphate from an impure sample. From its saturated solution, pure copper sulphate is produced as crystals through evaporation. Because soluble solids are separated from liquids using the evaporation process. For instance, the crystal of copper sulphate dissolves in water to create a copper sulphate solution. CuSO4 crystals are left behind after the water evaporates during evaporation.
Table of Content
Aim
Blue Vitriol Formula
Blue Vitriol Chemical Name
Preparation of Copper Sulphate Solution
Filtration and Concentration Process
Cooling Down the Hot, Saturated Solution
Drying and Crystal Separation
Result
Aim
To prepare crystals of Pure Copper Sulphate (CuSO4.5H2O) from a given impure sample of the blue vitriol.
Materials Required
A porcelain Bowl
A Funnel
An Evaporating Dish
A 400 ml Beaker
Crude Copper Sulphate Sample
A Policeman (Glass Rod).
Theory
Blue vitriol- The term "vitriol" refers to a class of chemical compounds made up of the sulphates of particular metals, originally copper or iron. The popular name for hydrated copper sulphate is blue vitriol.
Blue vitriol formula and chemical name—Copper sulphate CuSO4.5H2O or blue vitriol, is a salt that occurs naturally as big, translucent, deep-blue triclinic crystals. It also appears as a white powder in its anhydrous state.
The provided sample is first shaken with water. It is mixed with a few drops of diluted sulphuric acid to stop copper sulphate from hydrolysing. The sample's copper sulphate dissolves, while the insoluble contaminants are left behind. The remedy has been filtered. The filtrate is heated to the point of crystallisation before cooling. Crystals of copper sulphate split out while cooling.
Copper sulphate can be stirred with a silver spoon, not an iron spoon. Silver is less reactive than copper because it follows copper in the reactivity series. Therefore, no reaction occurs when stirring the CuSO4 solution with a silver spoon.
Procedure
Preparation of Copper Sulphate Solution:
Add minuscule quantities of the powdered crude copper sulphate to 25 to 30 ml of water.
To dissolve it, vigorously stir. Add the powdered sample multiple times until just a small amount is left after stirring for a while.
To make the solution transparent, add 2–3 ml of diluted sulphuric acid.
This keeps the copper sulphate from hydrolysing. This way, the copper sulphate solution is prepared.
Filtration and Concentration Process:
The concentration of the filtrate is done after filtering the solution to the crystallisation point. Impurities remain on the filter paper after filtering the solution and collecting the filtrate in a china container.
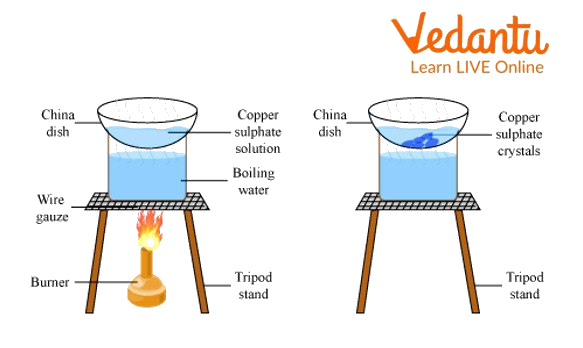
Heating of the Solution:
The solution should be heated in the china dish on a sand bath until it is roughly one-third of its original volume. With a glass rod, the solution is thoroughly swirled while it warms up to prevent the development of a crust on the dish's edge. If a crust forms, it is removed with a glass rod and dissolves into the solution. The dish's solution shouldn't be allowed to boil.
A drop of the solution should be removed from the glass rod's end and cooled by blowing. The glass rod will develop a crust or little crystals after the crystallisation point has been reached. Now cease heating and extinguish the burner. Place the heated, saturated solution in a dish for crystallisation.
Cooling Down the Hot, Saturated Solution:
Place the crystallisation plate containing the hot saturated solution atop a beaker full of water, and let it cool for a while. Crystals of copper sulphate will emerge with a deep blue colour. The crystallisation is finished in around 30 minutes.
Drying and Crystal Separation:
Carefully decant the mother liquor. With a tiny amount of cold water and a little ethyl alcohol, clean the crystals. Removing the crystals requires soaking filter paper in solution. The crystals are then transferred to a different filter paper, gently pressed between the paper's folds or spread out on a permeable plate to dry. Place the crystals in a dry test tube and cork it.
Crystallization process
Observations
On performing the crystallisation process, the water will evaporate, and you will receive pure crystals of CuSO4.
These pure crystals of CuSO4 are deep blue-blooded transparent in nature.
Result
The crystals of pure Copper sulphate blue colour having formula CuSO4.5H2O are obtained and are transparent.
Precautions
When concentrating, the filtrate should be gently heated and allowed to slowly evaporate.
Only the amount of filtrate needed to cause crystallization should be evaporated. Never let it get too hot or dry.
Avoid heating the solution too much.
The solution should be gently chilled without being disturbed. Never let it cool down quickly.
Three to four times, using a very small amount of washing liquid each time, wash the crystals in the liquid.
If the resulting crystals are exceedingly tiny, the solution has been concentrated beyond what is necessary for the crystallization step.
Lab Manual Questions
1. What method is utilized to isolate blue vitriol crystals from their impure samples?
Ans: Solids can be purified through crystallization, such as when alum crystals are separated from impure samples.
2. Which techniques are used to clean an impure sample of table salt?
Ans: Evaporation is the mechanism employed to separate impure salt from pure salt.
3. Which solvent is employed in the purification of benzoic acid and copper sulphate?
Ans: Water is the purifying agent used for copper sulphate and benzoic acid.
4. What happens if you put copper sulphate crystals in water?
Ans: A tiny quantity of heat energy is released during the dissolution process of pure copper sulphate when it is maintained in water.
Viva Questions
1. What happens when the crystals of blue vitriol are heated independently?
Ans: It turns into a white powder when it crystallizes because water is lost throughout the process.
2. Mother liquor: what is it?
Ans: The mother liquor is the liquid that remains after crystals are separated from a saturated solution.
3. What are the chemical formulas for crystals of green vitriol and potash alum?
Ans: The chemical formulas for crystals of green vitriol and potash alum are FeSO4.7H2O and (K2SO4.Al2(SO4)3.24H2O) respectively.
4. Why doesn't the hot, saturated solution cool down immediately?
Ans. Crystals enlarge as the saturated solution slowly cools. Instead of providing a massy substance with improper geometry, it aids in their better separation into units.
5. What does the word “seeding” mean?
Ans: Seeding occurs when a crystal of the same chemical is dissolved in a saturated solution. It facilitates fast crystal separation from a saturated solution.
6. What traits do crystals have?
Ans: The shape and geometry of crystals are clearly defined.
7. What does “saturated solution” mean?
Ans: A saturated solution is one in which, at a certain temperature, no more solute can dissolve.
8. What does the phrase "water of crystallization" refer to?
Ans: The water of crystallization is the specific quantity of water molecules that are present in proximity to one unit of the compound's formula.
9. How are crystals of pure substances obtained?
Ans: Crystals can develop straight from gas phase breakdown or from a pure melt.
10. What is solubility?
Ans: This is the amount of the solute needed to create a saturated solution in 100 g of solvent.
Practical Questions
From an impure sample, pure copper sulphate can be obtained using the process of
Fractional distillation
Centrifugation
Evaporation
Crystallization
Ans: From an impure sample, pure copper sulphate can be obtained using the process of crystallization.
Copper carbonate + ________________→ Copper sulphate + Water.
Copper sulphate
Sulphuric acid
Carbonic acid
Nitric acid
Ans: Copper carbonate + Sulphuric acid, → Copper sulphate + Water.
The most widely used solvent for crystallization is _______________.
Water
Alcohol
Saline water
Sulphuric water
Ans: The most widely used solvent for crystallization is water.
The solvent should dissolve a large amount of solute at ____________.
Cold temperature
Room temperature
Melting point
Boiling point
Ans: The solvent should dissolve a large amount of solute at room temperature.
The insoluble impurities from the solution during crystallization are removed by
Drying
Filtration
Heating
Cooling
Ans: The insoluble impurities from the solution during crystallization are removed by filtration.
When is supersaturation achieved?
When the solvent contains more solute.
When the solute contains more solvent.
When the solvent contains less solute.
When the solute contains more solvent.
Ans: Supersaturation is achieved when the solvent contains more solute.
Which of the processes is crystallization?
Solid-liquid separation
Solid-solid separation
Solid-gas separation
Liquid-gas separation
Ans: Solid-liquid separation is the process of crystallization.
Recrystallization process is used to_________
Purify chemicals
Dissolve crystals
Clean crystallizer
Continue the process of crystallization
Ans: The recycling process is used to purify chemicals.
Effect of concentration on crystallization is__________
An increase in concentration leads to large crystals.
An increase in concentration leads to small crystals.
A decrease in concentration leads to large crystals.
A decrease in concentration leads to small crystals.
Ans: The effect of concentration on crystallization leads to an increase in concentration leads to large crystals.
Supersaturated solution is unstable because__________
The concentration is higher than the equilibrium concentration.
Concentration is lower than the equilibrium concentration.
Concentration is equal to equilibrium concentration.
Contains impurities
Ans: A supersaturated solution is unstable because the concentration is higher than the equilibrium concentration.
Conclusion
From the above experiment, we can conclude that blue crystalline granules or powders are the typical forms of copper sulphate pentahydrate. The pentahydrate of copper(2+) sulphate is copper(II) sulphate, a crystallized bright blue substance. This tutorial helped us to learn all the different prospects of blue vitriol and pure copper sulphate.
FAQs on Class 11 Chemistry To Prepare Crystals Of Pure Copper Sulphate From A Given Impure Sample Of The Blue Vitriol Experiment
1. What are the most important steps to follow when preparing pure copper sulphate crystals from an impure sample for the Class 11 practical exam?
For the Class 11 Chemistry practical, the preparation of pure copper sulphate (CuSO₄·5H₂O) crystals involves these critical steps:
- Dissolution: Dissolve the impure copper sulphate sample in a minimum amount of water, adding a few drops of dilute sulphuric acid.
- Filtration: Filter the hot solution to remove any insoluble impurities.
- Concentration: Gently heat the filtrate in a china dish to evaporate water until the point of crystallisation is reached. This is tested by dipping a glass rod and seeing if a solid film forms upon blowing on it.
- Cooling: Allow the concentrated solution to cool down slowly and undisturbed. This allows for the formation of large, well-defined crystals.
- Separation and Drying: Carefully decant the mother liquor and separate the crystals. Wash them with a small amount of cold water and dry them between folds of filter paper.
2. What is the expected 1-mark viva question regarding the addition of dilute sulphuric acid during the copper sulphate crystallization experiment?
A very common viva question is: "Why is dilute sulphuric acid added while preparing the copper sulphate solution?" The correct answer is that it is added to prevent the hydrolysis of copper sulphate (CuSO₄) into basic copper sulphate, which is insoluble and would appear as an impurity.
3. Explain why hydrated copper sulphate (CuSO₄·5H₂O) is blue, but anhydrous copper sulphate (CuSO₄) is white. What is the role of water?
This is a high-order thinking question. Hydrated copper sulphate is blue because the five water molecules act as ligands surrounding the central Cu²⁺ ion. This arrangement causes the splitting of the d-orbitals of the copper ion. The salt can then absorb light in the orange-red region of the visible spectrum and appears blue, its complementary colour. In anhydrous CuSO₄, there are no water ligands, so no d-orbital splitting occurs, and it cannot absorb visible light, appearing white.
4. What is the fundamental principle behind using crystallization to purify an impure sample of copper sulphate?
The principle of crystallization is based on the difference in solubility. Copper sulphate is highly soluble in hot water but much less soluble in cold water. Most impurities, however, are either insoluble in water (and are filtered off) or are present in much smaller amounts and remain dissolved in the solution (the mother liquor) even after cooling. This allows the pure copper sulphate to crystallize out from the concentrated solution upon cooling.
5. What might be the reason if crystals do not form after cooling the concentrated copper sulphate solution, and what is the remedy?
If crystals do not form upon cooling, the solution is likely in a supersaturated state. This means it holds more dissolved solute than it normally would at that temperature without crystallizing. The remedy is to induce crystallization by:
- Seeding: Adding a single, tiny crystal of pure copper sulphate (a 'seed crystal') to the solution.
- Scratching: Gently scratching the inside wall of the beaker with a glass rod to provide a rough surface for crystal growth to begin.
6. How would the experimental procedure for crystallizing benzoic acid differ from that of copper sulphate?
The primary difference lies in the choice of solvent and solubility properties. While both can be purified from an impure sample, the key differences in procedure are:
- Solvent: Copper sulphate is crystallized from an aqueous solution. Benzoic acid is sparingly soluble in cold water but highly soluble in hot water, making hot water the ideal solvent for its crystallization.
- Hydrolysis: Dilute acid is added for copper sulphate to prevent hydrolysis. This step is not required for benzoic acid.
7. For the Class 11 Chemistry exam, what are two crucial precautions to observe while preparing copper sulphate crystals?
To ensure good results and safety, students must observe the following precautions:
- Avoid Overheating: Do not heat the solution to complete dryness during concentration. This would cause the hydrated blue crystals (CuSO₄·5H₂O) to turn into white anhydrous powder (CuSO₄) and would also bake the impurities along with the product.
- Ensure Slow Cooling: The concentrated solution must be cooled slowly and without disturbance. Rapid cooling results in the formation of very small, poorly-defined crystals instead of large, pure ones.
























