




An Overview of Class 11 Chemistry Viva Questions With Answers On A Determination Of Melting Point Experiment
Introduction
To determine an object's purity, the melting points of several elements are measured. The melting range of an incredibly pure substance will be one or two degrees. The melting range is higher and smaller than the initial impure one and is typically depressed and widened by impure chemicals, which have a considerably higher range. A certain temperature change causes the melting to happen, but the molecule's structure also plays a role. Varying compounds have different melting characteristics precisely because of this.
Table of Contents
Aim
Apparatus required
Theory Procedure
Observations
Result
Precautions
Aim
To figure out the melting point of a given solid substance.
Apparatus Required
Given solid substance
Aluminium block
Clamped stand
Capillary tube
Tripod
Thermometer
Benzoic Acid
Kerosene burner
Theory
Melting is the process of a compound changing from a solid to a liquid state when heated, and the melting point is the temperature at which a solid melts in its purest form. The determination of the melting point aids in the identification of the compound because every pure solid has a characteristic melting point. The melting point of the solid is lowered by the presence of impurities. Thus, a compound's melting point can be used to determine its purity.
Procedure
Take a 5- to 6-cm-long fine capillary. Seal the capillary tube's one end by placing the end horizontally into the extreme edge of a small, steady Bunsen flame for a short period of time while rotating the capillary.
On a porous plate, place a tiny amount of the substance whose melting point needs to be determined and grind it with a spatula.
By inserting the capillary tube's open end into the powdered compound and gently spinning it, you can introduce the powdered compound into the tube.
To make the compound sink into the closed end, gently tap the capillary tube against the porous plate. Three to four times, repeat the introducing and tapping steps.
To attach the capillary to the thermometer's lower end, moisten the bulb with liquid paraffin or concentrated sulphuric acid.
Place the thermometer and capillary tube in the melting point device such that the closed end of the capillary tube stays below the liquid paraffin's surface. The melting point apparatus should contain at least two thirds of its volume of liquid paraffin.
Now, gently heat the beaker and record the temperature every so often. Finally, record the temperature at which the compound begins to melt and finishes melting.
With a new capillary tube and new amounts of the material, repeat the experiment.
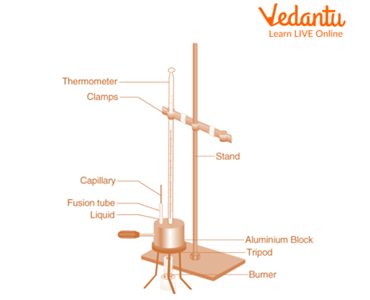
Determination of Melting Point
Observation
The temperature at which the unknown substance starts to melt is denoted as 119 °C
The temperature at which a substance will totally melt is denoted as 124 °C
Unknown substance's melting point is \[\dfrac{{119 + 124}}{2} = 121.5^\circ C.\]
Results
The melting point of the given compound =121.5 °C.
Precautions
Make sure the benzoic acid and naphthalene samples are dry and pulverised.
Keep the thermometer and capillary tube at the same height.
Avoid leaving any air spaces when packing the powder into the capillary tube.
Lab Manual Questions
1. Ionic compounds have higher melting points for what reason?
Ans. Because of the strong electrostatic forces that interact between ions, ionic compounds have high melting temperatures.
2. How can we characterise a substance using melting points?
Ans. The melting point of a pure substance will be sharp, meaning that the compound melts fully within a range of 2 °C.
3. Ionic chemicals crystallise for what reason?
Ans. Ions are grouped in precise three-dimensional ways in ionic compounds, which makes them crystalline in nature.
4. Compounds that are nonpolar are volatile. mention a reason.
Ans. Non-polar substances are volatile because there are weak van der Waals interactions between their molecules.
Viva Questions
1. What is the melting point?
Ans. It is described as the constant temperature at which a substance can exist in both its solid and liquid states.
2. How can finding the melting point inform us of the purity of the compound?
Ans. The melting point reveals a substance's purity. A substance's melting point is typically lowered if it contains moisture or another contaminant. A pure substance is one that has a narrow melting point.
3. The acute melting point is what?
Ans. A solid's melting point is considered acute if it entirely melts within a 1 °C range.
4. Why is the melting point of pure solids so high?
Ans. A pure solid melts at a constant temperature because it has the same force of attraction between particles everywhere.
5. How do impurities affect the melting point of solids?
Ans. Solids with impurities have lower melting points.
6. Can we directly heat the capillary tube to find its melting point?
Ans. No, direct heating would provide a quick and uneven heating.
7. Can any other liquid be used to calculate the melting point of liquid paraffin?
Ans. Yes, you may find the melting point using concentrated H2S04 or silicone oils.
8. Why does benzamide have a higher melting point than acetamide?
Ans. The functional group of benzamide and acetamide is the same, however benzamide has a larger molecular mass than acetamide. Because of this, benzamide has greater melting point and stronger intermolecular pressures.
9. Why do various substances' melting points vary?
Ans. Intermolecular forces that occur in the solid-state affect melting point. Since the intensity of the intermolecular interactions varies among different substances, so do their melting points.
10. What temperature does water reach its maximum density?
Ans. The maximum density of water is reached at 4 °C.
Practical Based Questions:
1. The compound's purity is verified by _______.
Its melting and boiling point
Chromatographic technique
Spectroscopy
All of the above
Ans. The compound's purity is verified by its melting and boiling point, chromatographic technique, and spectroscopy.
2. Among the following assertions, pick the ones that are true.
Melting is the process of turning a solid into a liquid, and freezing is the opposite.
Freezing is the process of turning a solid into a liquid, and melting is the opposite.
Melting is the process of turning a liquid into a solid, whereas freezing is the opposite.
None of the above
Ans. Melting is the process of turning a solid into a liquid, and freezing is the opposite.
3. The temperature at which solid and liquid coincide in equilibrium is called ______.
Melting point of liquid
Freezing point of liquid
Freezing point of solid
All of the above
Ans. The temperature at which solid and liquid coincide in equilibrium is called freezing point of liquid.
4. Among the following statements, pick the ones that are false.
Every pure solid crystalline substance has a certain melting point that is typical to it.
A sample of impure material has a varied melting point.
The melting points of two separate pure substances coincide.
The test for a solid substance's purity is its melting point.
Ans. The melting points of two separate pure substances coincide.
5. The temperature _____ when the solid and liquid phases are in equilibrium.
Increases gradually
Decreases gradually
Remains constant
None of the above
Ans. The temperature remains constant when the solid and liquid phases are in equilibrium.
6. Ice melts at a temperature of _____.
0 °C
100 °C
4 °C
-4 °C
Ans. Ice melts at a temperature of 0 °C.
7. The definition of molar heat of fusion is.
Energy needs to melt one gram of solid.
Energy needs to melt one mole of solid.
Energy needs to melt one kilogram of solid.
Energy needs to melt ten moles of solid.
Ans. The definition of molar heat of fusion is energy needed to melt one mole of solid.
8. At 100 °C, how does water behave?
Solid
Vapour
Liquid
Liquid and vapour
Ans. At 100 °C, water behaves as vapour.
9. What is the water's molar heat of fusion?
1.3
0.84
7.61
6.01
Ans. The water's molar heat of fusion is 6.01.
10. What is the mercury's melting point?
357 °C
-39 °C
0 °C
100 °C
Ans. The mercury's melting point is -39 °C.
Conclusion
People can better grasp a material's physical and chemical qualities by knowing its melting point. Any substance's melting point is influenced by a wide range of variables, including the force of attraction, any impurities present, and the size and structure of the molecules.
FAQs on Class 11 Chemistry Viva Questions With Answers On A Determination Of Melting Point Experiment
1. What is the primary principle behind the determination of the melting point of an organic compound, a key experiment for the Class 11 practical exam?
The experiment is based on the core principle that a pure crystalline solid melts at a sharp, specific temperature when heated. The melting point is technically the temperature at which the solid and liquid phases of a substance coexist in equilibrium at atmospheric pressure. This characteristic physical property is fundamentally used to identify an unknown organic compound and to ascertain its purity.
2. What are some important precautions students must take while performing the melting point experiment to get accurate results for the exam?
To ensure an accurate determination of melting point, which is crucial for scoring well in the practical exam, students must observe the following precautions:
- The organic compound should be finely powdered and packed tightly into the capillary tube to a height of about 2-3 mm.
- The heating of the liquid bath (e.g., liquid paraffin) must be slow and uniform, especially when the temperature is close to the expected melting point.
- The thermometer and the capillary tube should be arranged so their lower ends are at the same level.
- The liquid bath should be stirred continuously to maintain a uniform temperature throughout.
- Record the temperature at which the substance starts to melt (T1) and the temperature at which it completely melts (T2). The melting point range is T1-T2.
3. How do impurities affect the melting point of a compound, and why is this a frequently asked question in exams?
Impurities disrupt the uniform crystal lattice structure of a solid. This weakens the intermolecular forces, causing the substance to melt at a lower temperature than the pure compound. Furthermore, the melting process occurs over a wider range of temperatures instead of at a sharp, fixed point. This concept is a favourite exam question because it directly tests a student's understanding of how physical properties indicate purity. For instance, if a pure compound has a sharp melting point of 120°C, an impure sample might melt over a broad range like 115-118°C.
4. What are the essential components of the apparatus used for determining melting point, as expected in a diagram-based question?
The standard apparatus for determining melting point, often asked in diagrams and viva voce, consists of the following essential components:
- Thiele's Tube: A specially designed glass tube that uses convection currents to ensure uniform heating of the liquid bath.
- Liquid Bath: A liquid with a high boiling point and thermal stability, such as liquid paraffin or concentrated sulphuric acid.
- Capillary Tube: A thin glass tube, sealed at one end, which holds the powdered organic substance.
- Thermometer: To accurately measure the temperature of the liquid bath.
- Burner and Stand: To provide a source of controlled heat.
5. Why is it critical to heat the apparatus slowly, especially when approaching the actual melting point?
It is critical to heat the apparatus slowly to ensure thermal equilibrium. If heated too quickly, the temperature of the liquid bath will rise faster than the temperature of the sample inside the capillary tube due to a time lag in heat transfer. Consequently, the thermometer will show a higher temperature than the actual temperature at which the substance melts. This leads to an erroneously high and inaccurate melting point reading. Slow and steady heating ensures that the temperature of the sample and the thermometer are identical at all times.
6. What is the precise definition of 'melting point' and 'melting point range' expected for the CBSE Class 11 Chemistry exam?
In the context of the CBSE exam, these terms have specific meanings:
- Melting Point: It is the specific temperature at which a solid substance transitions into its liquid state at one atmosphere of pressure. At this temperature, the solid and liquid phases are in equilibrium. A sharp melting point is a characteristic of a pure substance.
- Melting Point Range: This is the temperature span from when the substance first begins to liquefy to when it becomes completely liquid. For a pure compound, this range is very narrow (0.5-1°C), whereas for an impure compound, the range is significantly broader.
7. How are intermolecular forces of attraction related to the melting point of an organic compound? This is an important concept for HOTS questions.
The melting point of a compound is a direct indicator of the strength of its intermolecular forces (the forces holding molecules together in the crystal lattice).
- Stronger Forces: Compounds with strong forces, such as hydrogen bonding or ionic interactions, require more thermal energy to break apart the lattice. Thus, they have high melting points.
- Weaker Forces: Compounds with only weak van der Waals forces are easier to melt as less energy is needed to overcome these forces. They exhibit low melting points. This principle is crucial for answering questions that require comparing the melting points of different substances.
























