Chemical Bonding and Molecular Structure Class 11 Extra Questions and Answers Free PDF Download
FAQs on CBSE Important Questions for Class 11 Chemistry Chemical Bonding and Molecular Structure - 2025-26
1. What are the most important topics in Class 11 Chemistry Chapter 4 for the 2025-26 exams?
For the CBSE 2025-26 exams, the most important topics from Chemical Bonding and Molecular Structure that are frequently asked include:
- VSEPR Theory: Crucial for questions on predicting the geometry and shape of simple molecules.
- Hybridization: Understanding the types (sp, sp², sp³) and their role in forming covalent bonds is a key area.
- Molecular Orbital Theory (MOT): Essential for explaining the magnetic properties and stability of diatomic molecules like O₂, N₂, and F₂.
- Bond Parameters: Exam questions often test the concepts of bond angle, bond length, bond enthalpy, and bond order.
- Dipole Moment: Its application in determining the polarity of molecules like H₂O, NH₃, and CO₂ is an important question type.
2. Which types of questions are commonly expected from this chapter for 3 or 5 marks?
For higher marks in your Class 11 Chemistry exam, expect questions that require detailed explanations and application of concepts.
- 5-Mark Questions: Typically involve a detailed explanation of Molecular Orbital Theory, including drawing the energy level diagram for molecules like N₂ or O₂, or explaining all the postulates of VSEPR theory with multiple examples.
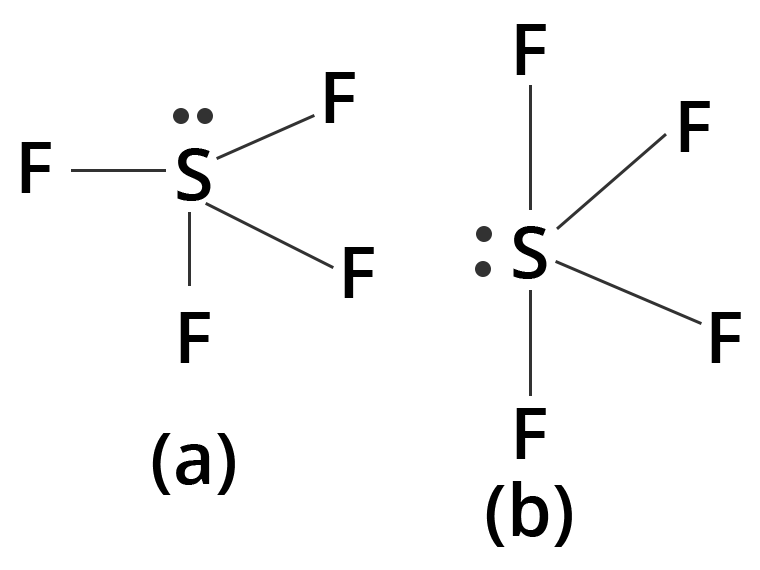
- 3-Mark Questions: These often ask you to predict and draw the shapes of 2-3 molecules using VSEPR theory (e.g., NH₃, H₂O, SF₄), explain a specific type of hybridization with an example, or differentiate between sigma and pi bonds.
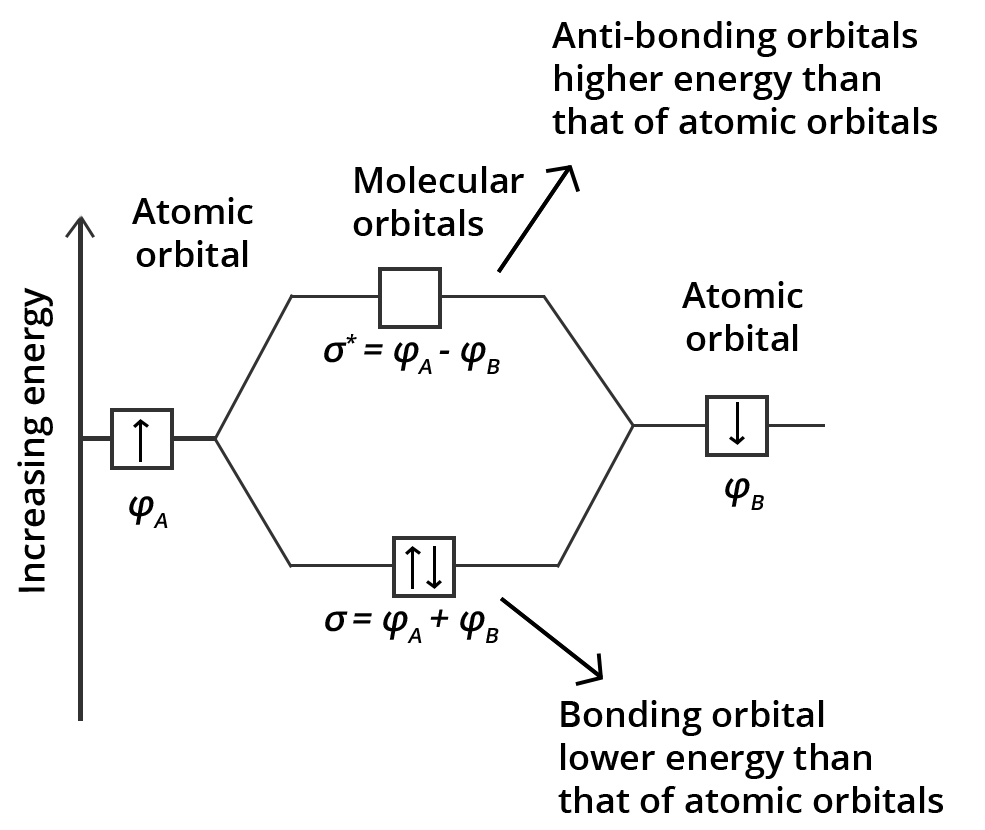
3. How does Molecular Orbital Theory (MOT) explain why the He₂ molecule does not exist?
This is a classic higher-order thinking question frequently asked in exams. According to MOT, in a hypothetical He₂ molecule (with 4 electrons), two electrons would fill the bonding molecular orbital (σ1s) and two would fill the antibonding molecular orbital (σ*1s). The bond order is then calculated as ½ (Number of bonding electrons - Number of antibonding electrons). For He₂, this would be ½ (2 - 2) = 0. A bond order of zero signifies that no stable bond is formed, which explains why the He₂ molecule does not exist.
4. What is the fundamental difference between Valence Bond Theory (VBT) and Molecular Orbital Theory (MOT)?
This is an important comparative question for exams. While both theories explain covalent bonding, their core assumptions differ:
- Valence Bond Theory (VBT): This theory states that atomic orbitals of individual atoms overlap to form covalent bonds. The electrons remain localized to their original atomic orbitals. VBT is particularly effective in explaining the geometry of molecules through the concept of hybridization.
- Molecular Orbital Theory (MOT): This theory proposes that atomic orbitals combine to form a new set of molecular orbitals that belong to the molecule as a whole. Electrons are delocalized over the entire molecule. MOT is superior in explaining properties that VBT cannot, such as the paramagnetic nature of the O₂ molecule.
5. How can you determine the hybridization of the central atom in molecules like CH₄, BeCl₂, and PCl₅ for exams?
A straightforward method for exam purposes is to count the total number of sigma bonds and lone pairs of electrons around the central atom.
- CH₄ (Methane): The central carbon atom forms 4 sigma bonds with hydrogen and has 0 lone pairs. The total is 4, which corresponds to sp³ hybridization.
- BeCl₂ (Beryllium Chloride): The central beryllium atom forms 2 sigma bonds with chlorine and has 0 lone pairs. The total is 2, corresponding to sp hybridization.
- PCl₅ (Phosphorus Pentachloride): The central phosphorus atom forms 5 sigma bonds with chlorine and has 0 lone pairs. The total is 5, corresponding to sp³d hybridization.
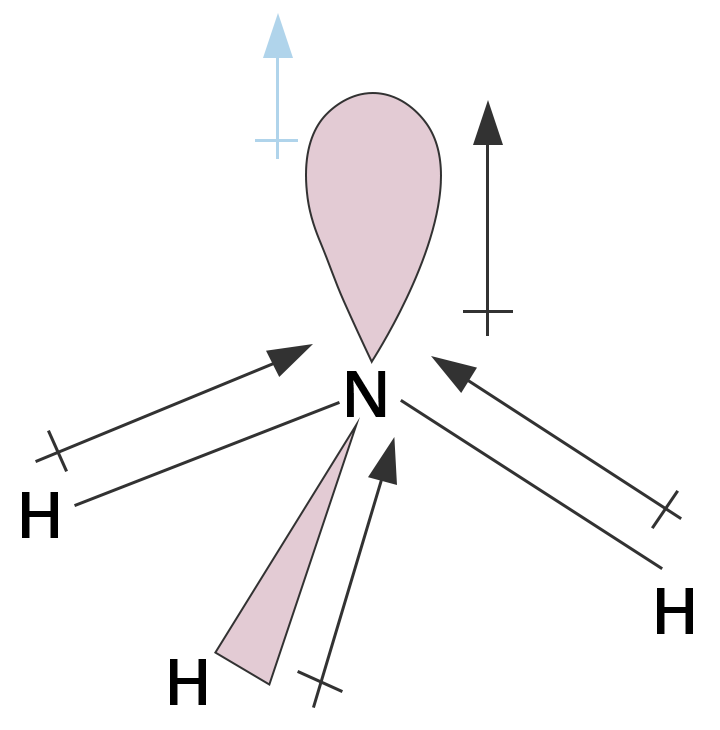
6. Why is the bond angle in H₂O (104.5°) smaller than the bond angle in NH₃ (107°), even though both have central atoms with sp³ hybridization?
This is a crucial conceptual question based on VSEPR theory. The difference in bond angle is due to the number of lone pairs on the central atom. According to VSEPR, the order of repulsion is: lone pair-lone pair > lone pair-bond pair > bond pair-bond pair.
- In NH₃, the central nitrogen atom has one lone pair and three bond pairs. The repulsion between the lone pair and bond pairs compresses the angle slightly from the ideal 109.5°.
- In H₂O, the central oxygen atom has two lone pairs and two bond pairs. The much stronger repulsion between the two lone pairs pushes the bonding pairs even closer together, resulting in a significantly smaller bond angle.
7. What is dipole moment and how is it used to predict if a molecule like CO₂ is polar or non-polar?
Dipole moment is a measure of the net polarity of a molecule, arising from the separation of positive and negative charges. For exam questions, it's important to consider both bond polarity and molecular geometry. In CO₂, the C=O bond is polar because oxygen is more electronegative than carbon. However, the molecule has a symmetrical, linear shape (O=C=O). The two bond dipoles are equal in magnitude but point in opposite directions. As a result, they cancel each other out, leading to a net dipole moment of zero. Therefore, CO₂ is a non-polar molecule despite having polar bonds.
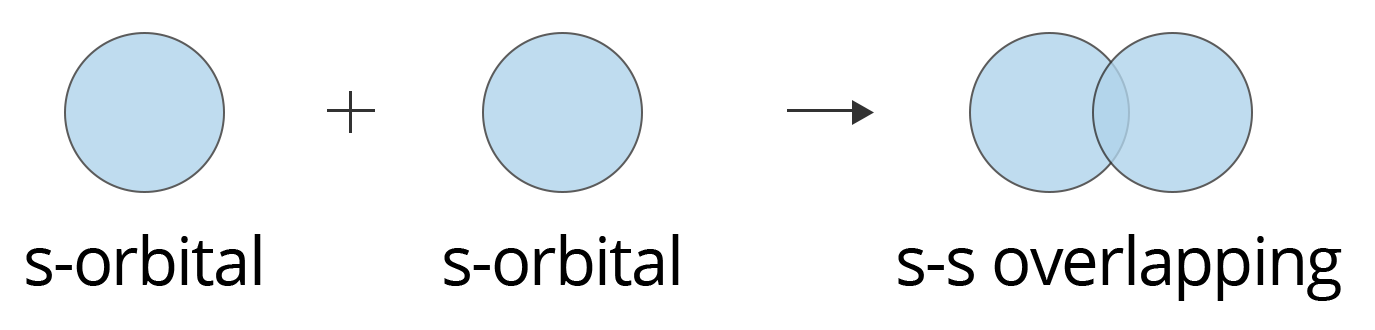
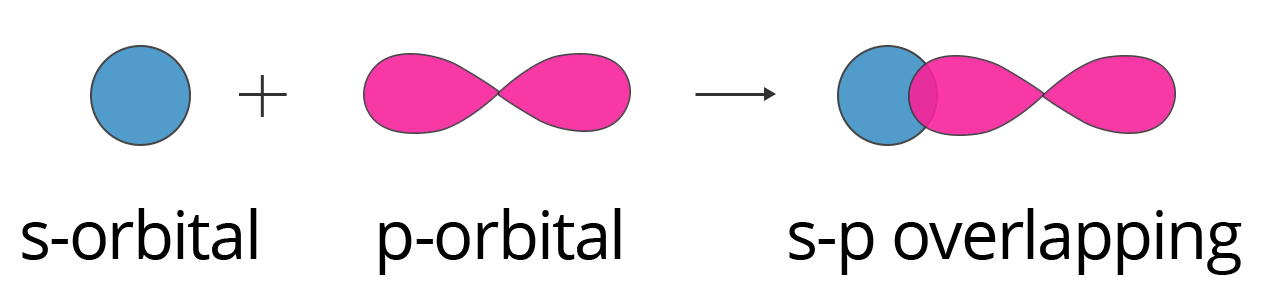
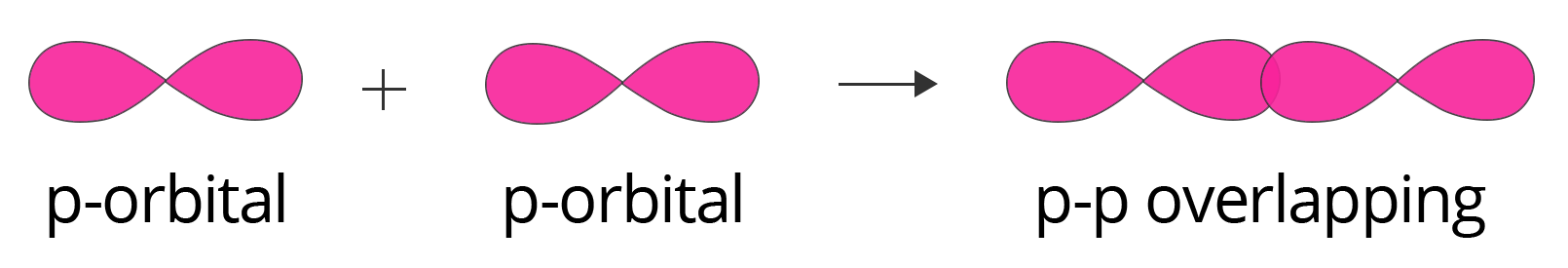
8. Explain why a sigma (σ) bond is considered stronger than a pi (π) bond.
The strength of a covalent bond is directly related to the extent of orbital overlap. This is a key point for exam answers.
- A sigma (σ) bond is formed by the direct, head-on (axial) overlap of atomic orbitals. This allows for a large area of overlap along the axis connecting the two nuclei, creating a strong bond.
- A pi (π) bond is formed by the sideways (lateral) overlap of unhybridized p-orbitals. This overlap is less effective as it occurs above and below the internuclear axis.