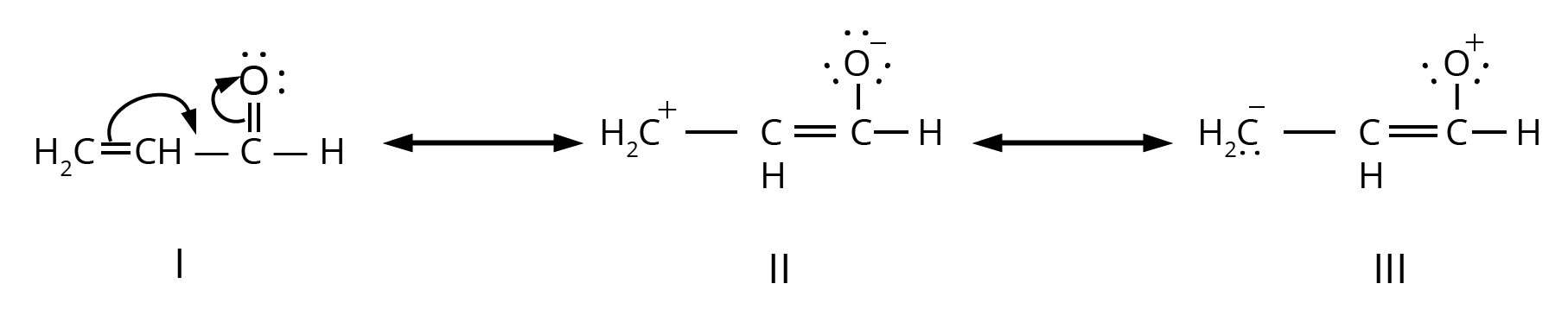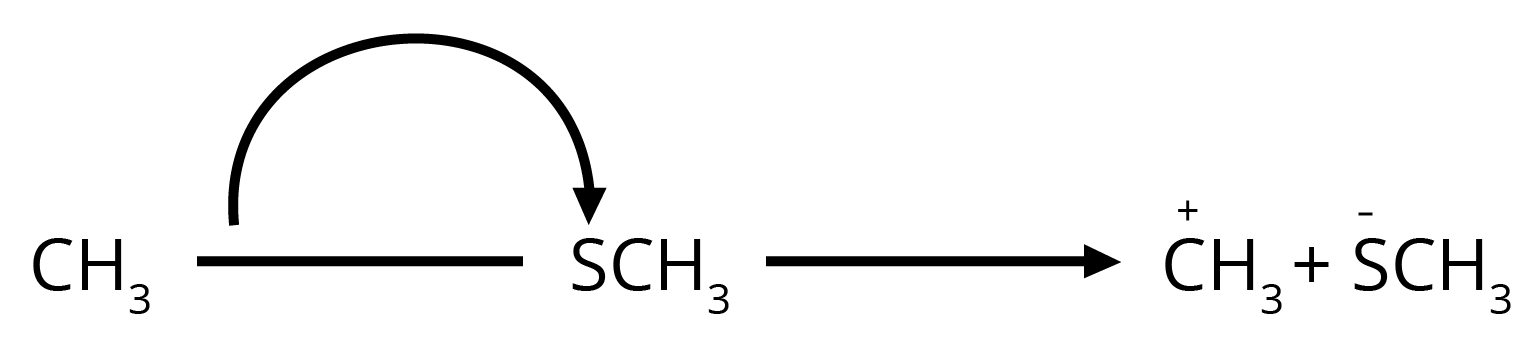Organic Chemistry - Some Basic Principles and Techniques Class 11 important questions with answers PDF download
FAQs on CBSE Important Questions for Class 11 Chemistry Organic Chemistry - Some Basic Principles and Techniques - 2025-26
1. How important is Chapter 8, Organic Chemistry - Some Basic Principles and Techniques, for my Class 11 exams?
This chapter is extremely important as it forms the foundation for all of organic chemistry. You can expect a mix of questions, including short-answer questions (1-2 marks) for definitions and long-answer questions (3-5 marks) involving nomenclature, isomerism, and reaction mechanisms. Mastering this chapter is crucial for scoring well in the organic chemistry section.
2. What are the high-weightage topics I should focus on in this chapter for the 2025-26 CBSE exam?
For your final exams, it's wise to concentrate on the following high-scoring areas:
- IUPAC Nomenclature: Naming organic compounds and drawing structures from names.
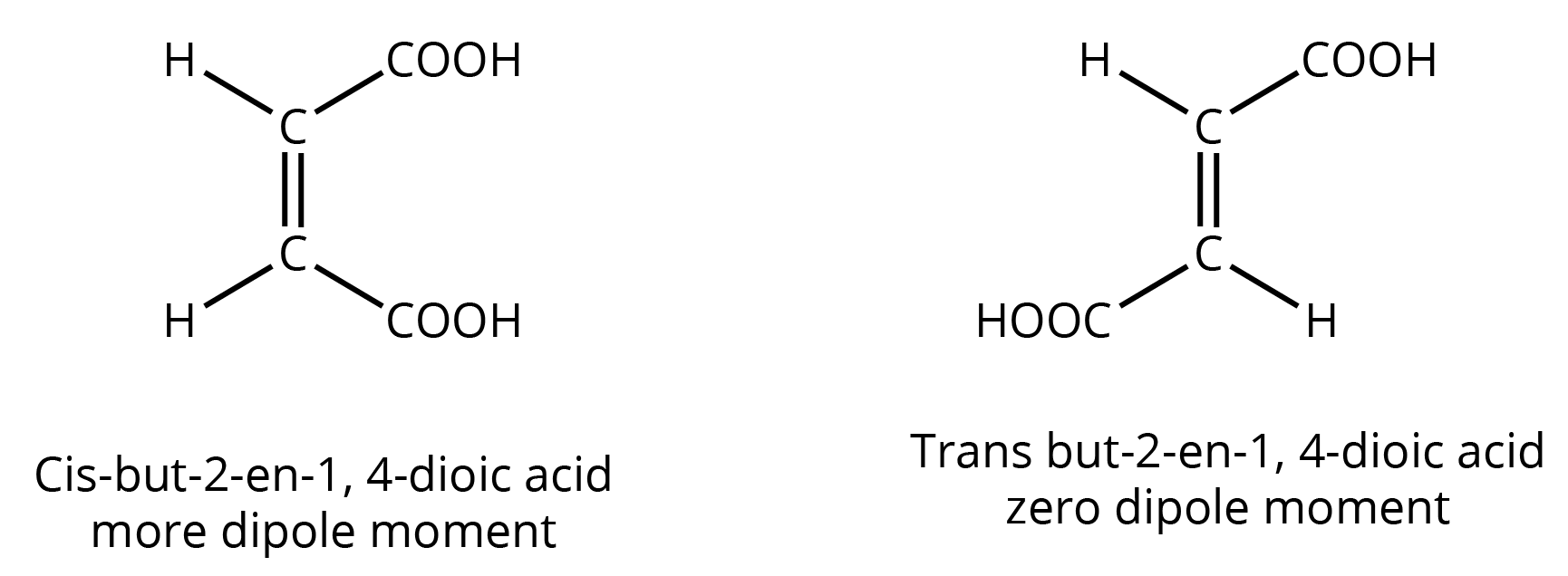
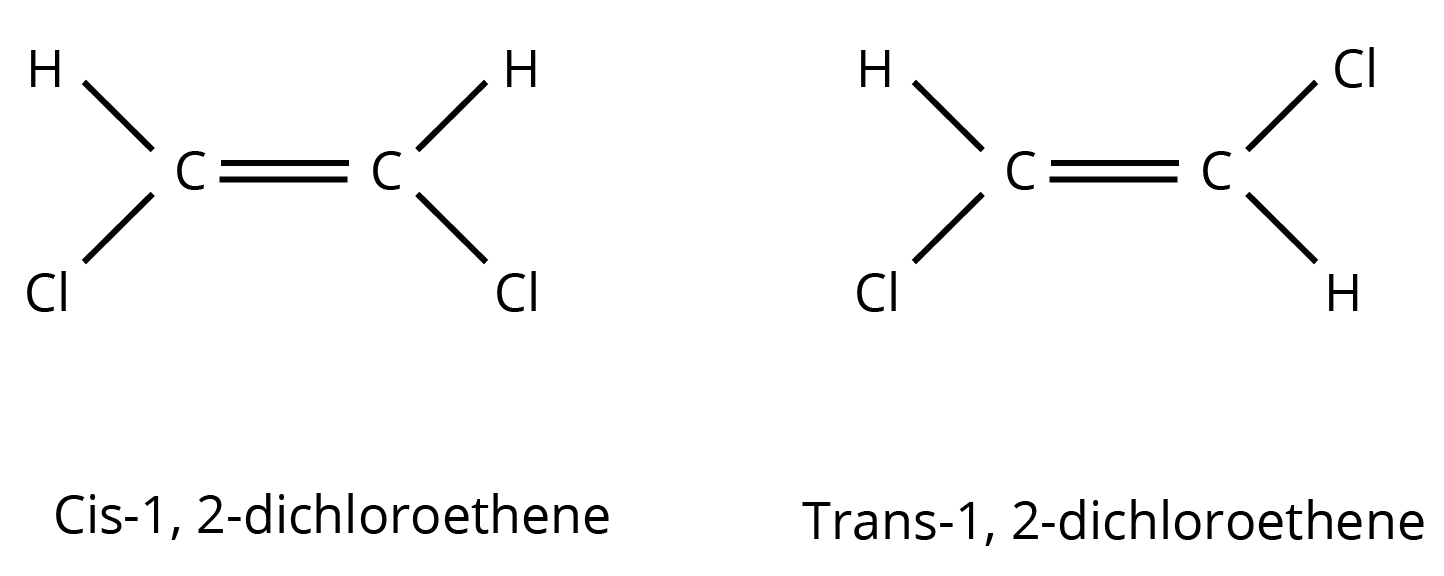
- Isomerism: Especially structural isomerism (chain, position, functional) and stereoisomerism.
- Fundamental Concepts: Understanding electronic effects like the inductive effect and resonance to explain stability.
- Purification Techniques: The principles behind methods like distillation and chromatography.
3. What kind of questions are typically asked from the isomerism and electronic effects sections?
Examiners often test your conceptual understanding. Expect questions like:
- Identifying the type of isomerism between two given compounds.
- Drawing all possible structural isomers for a given molecular formula.
- Arranging carbocations or free radicals in order of increasing or decreasing stability, and justifying your answer using resonance or inductive effects.
- Explaining why a particular molecule is more stable or reactive than another.
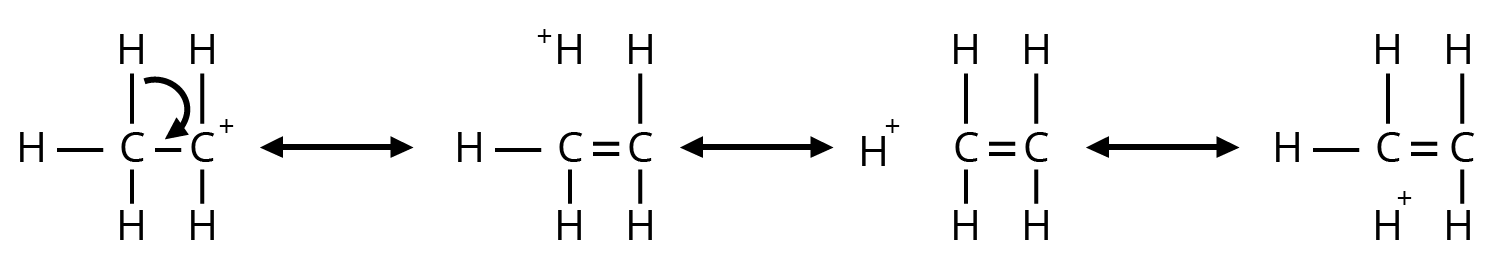
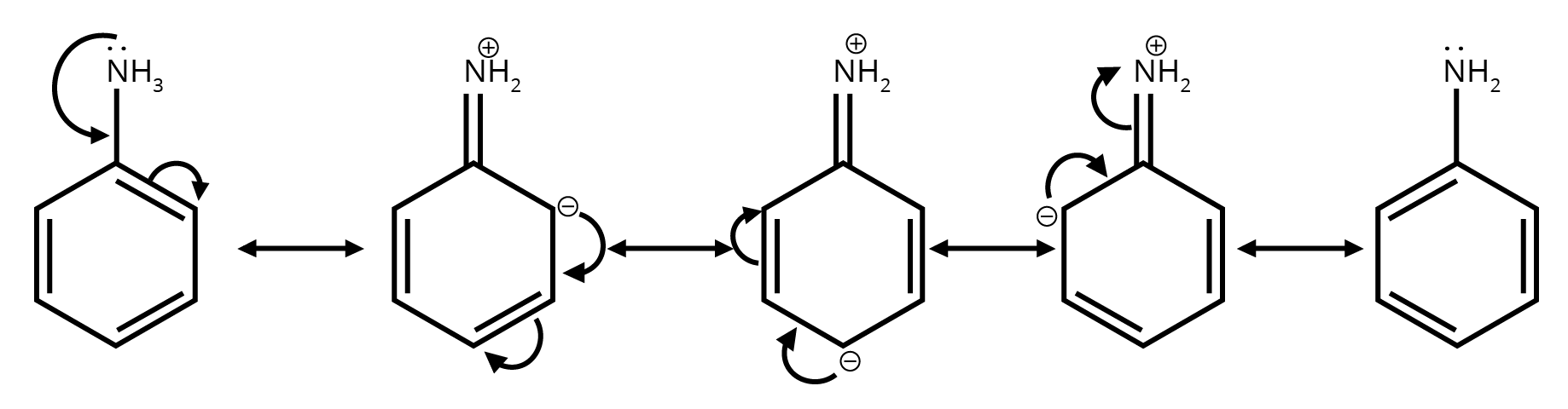
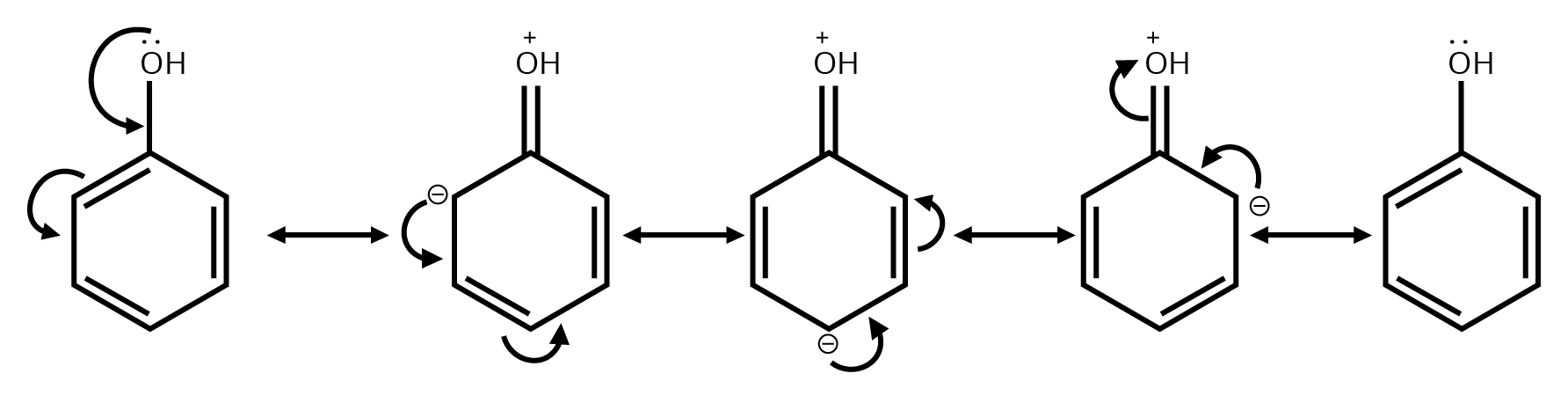
4. Why is understanding resonance so critical for answering questions from this chapter?
Understanding resonance is not just about drawing structures; it's about predicting stability. In exams, you'll be asked to compare the stability of different molecules or reaction intermediates. The key principle is that molecules with more resonance structures are generally more stable because the charge is spread out (delocalised). This concept is essential for explaining reaction pathways and outcomes.
5. What are the common mistakes students make in IUPAC nomenclature questions?
Students often lose marks due to simple errors. The most common mistakes are:
- Not selecting the longest possible carbon chain.
- Incorrectly numbering the chain, especially in compounds with multiple functional groups.
- Forgetting to alphabetise the substituent names.
- Using incorrect prefixes or suffixes for functional groups.
Practising regularly is the best way to avoid these mistakes.
6. How does mastering Class 11 Organic Chemistry help in Class 12?
This chapter is the absolute backbone of Class 12 organic chemistry. All the chapters in Class 12, such as Haloalkanes, Alcohols, and Aldehydes, rely heavily on the concepts of electrophiles, nucleophiles, reaction intermediates, and electronic effects (inductive, resonance) that you learn here. A strong foundation in this chapter makes understanding the complex reactions in Class 12 much easier.
7. For a 5-mark question, what level of detail is expected when explaining a purification technique?
For a high-scoring question on a technique like fractional distillation, you should be able to explain:
- The principle behind the method (e.g., separating liquids with close boiling points).
- A neat, labelled diagram of the apparatus.
- A brief description of the procedure.
- At least one practical application, for example, separating crude oil components.
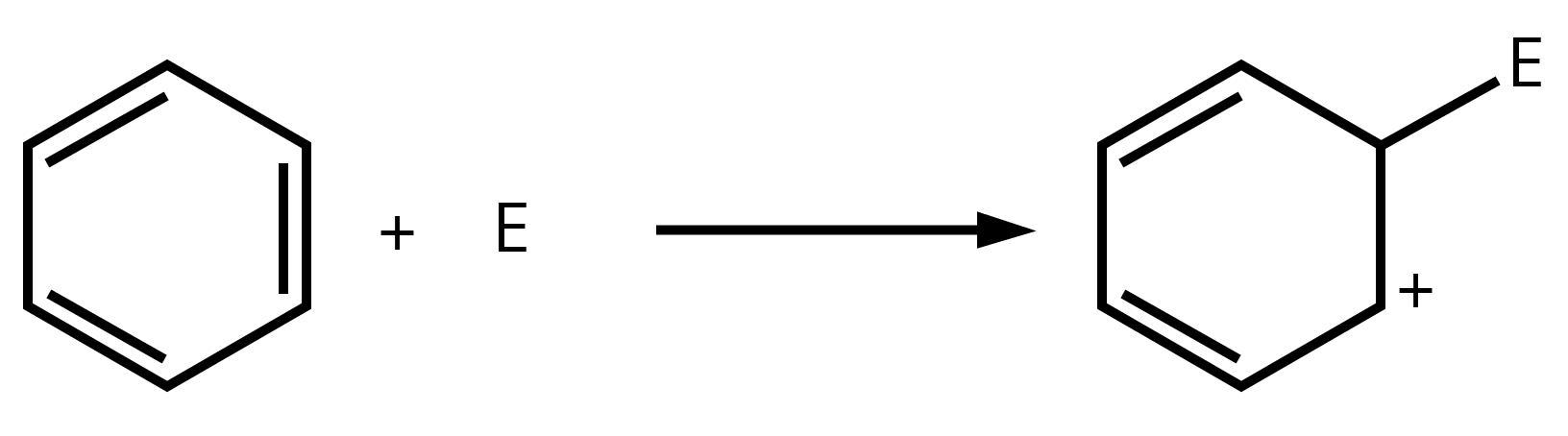
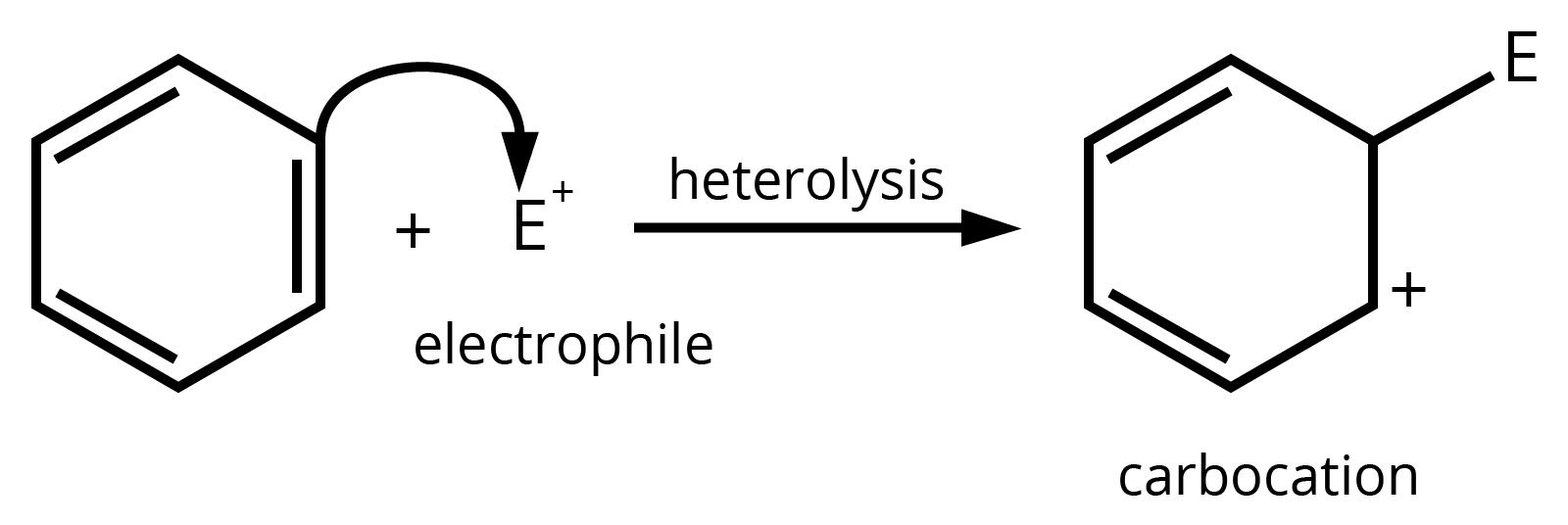
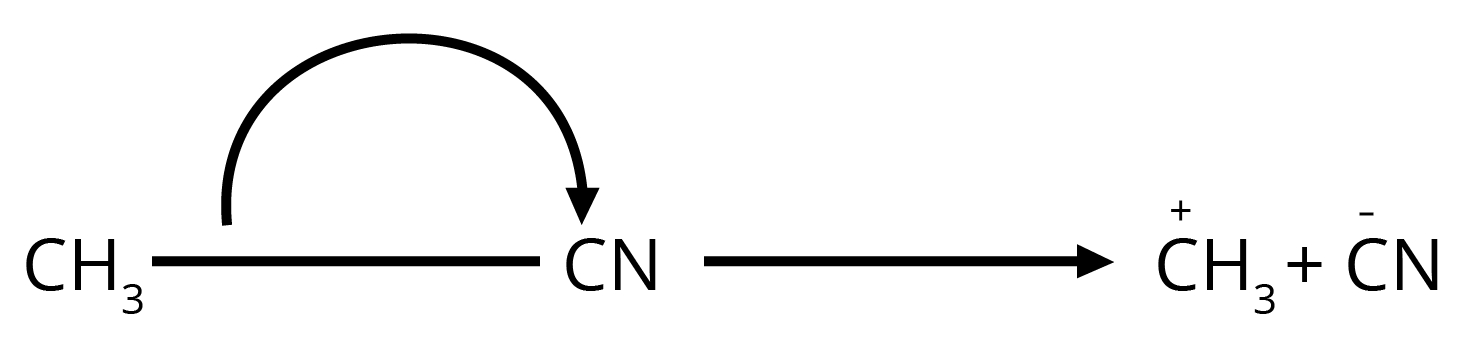
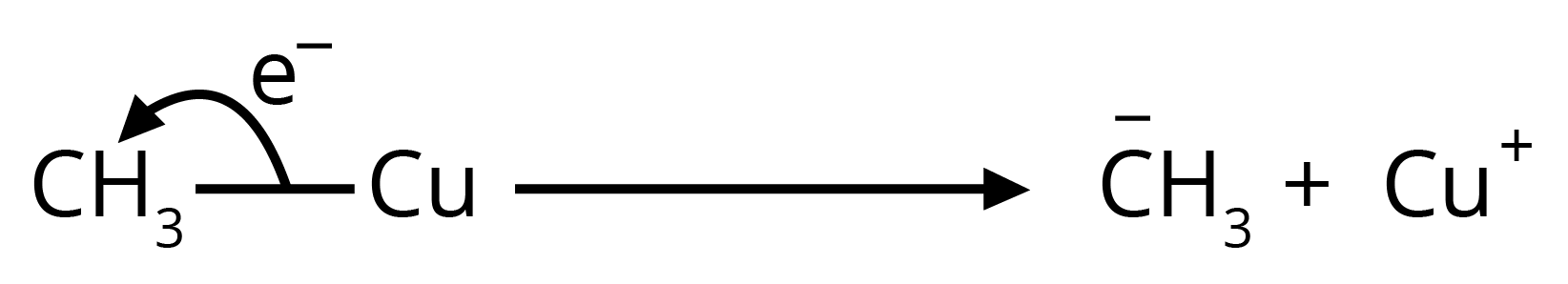
8. How can I quickly identify nucleophiles and electrophiles in a reaction?
It's a simple concept that's very important for exams. Think of it this way:
- Nucleophiles are 'nucleus-loving' and are electron-rich. Look for species with a negative charge (like OH⁻, Cl⁻) or a lone pair of electrons (like H₂O, NH₃).
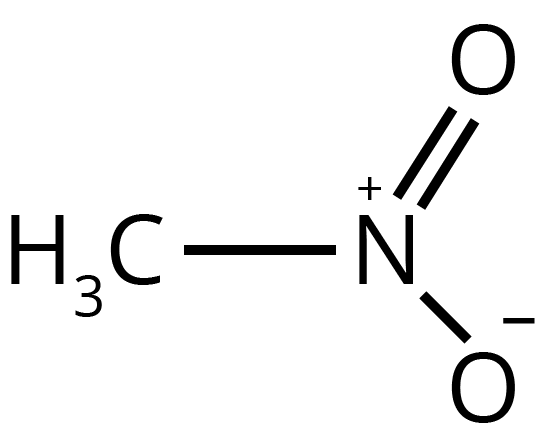
- Electrophiles are 'electron-loving' and are electron-deficient. Look for species with a positive charge (like H⁺, NO₂⁺) or an incomplete octet (like BF₃).
Identifying these correctly is the first step to solving any reaction mechanism question.