




Step-by-Step Mechanism of Allylic Substitution Reactions
The Allylic Substitution Reaction is a key transformation in organic chemistry relevant for JEE Main, as it involves the replacement of a leaving group or hydrogen at the allylic position of alkenes by another group under specific conditions. Understanding allylic substitution reaction mechanisms, such as SN1', SN2', and free radical processes, is crucial for predicting products and solving typical exam questions. This topic bridges fundamental reactivity with practical synthesis and industrial applications.
Core Concepts: What Is an Allylic Substitution Reaction?
In allylic substitution, a group on the carbon atom directly next to a carbon-carbon double bond (the allylic position) is replaced by a nucleophile, electrophile or radical. The stability at this site, due to resonance of the allylic intermediate, makes these reactions faster and often more selective than other substitutions. Exam questions often emphasise recognising allylic positions and differentiating these reactions from acyl or benzylic substitutions.
A typical example is the reaction of an allylic halide (like allyl chloride) with a nucleophile such as hydroxide ion to form an alcohol. JEE tests both conceptual and application skills in predicting the product structure, mechanism, and resonance stabilization of intermediates for nucleophilic and free radical allylic substitutions.
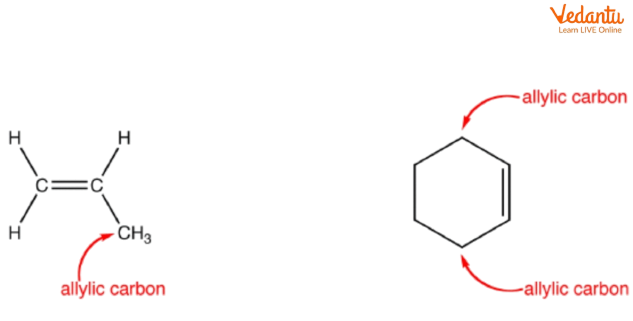
Identifying the Allylic Position in Alkenes
The allylic carbon is one carbon away from a double bond. To find the allylic position, locate the C=C double bond and mark each adjacent carbon atom. These positions are highly reactive in many substitution mechanisms due to delocalisation (resonance) of the resulting carbocation or radical intermediates. Awareness of this pattern speeds up major product identification in MCQs.
Types of Allylic Substitution: SN1', SN2', Electrophilic and Free Radical
There are several mechanistic variants for allylic substitution reaction in organic chemistry, each relevant for different conditions and reagents. Examining and comparing these helps with mechanism-based MCQs and numericals. The prime notation (') signals substitution at the allylic carbon with rearrangement via resonance.
| Type | Mechanism | Key Features | Typical Example |
|---|---|---|---|
| SN1' | Two-step. Allylic carbocation stabilized by resonance. | Racemic mixture; rearrangement possible. | Allyl bromide + H2O → Allyl alcohol |
| SN2' | Single-step. Concerted attack and rearrangement. | Inversion of configuration; major product via resonance. | Allyl chloride + CN- → Allyl cyanide |
| Free Radical (Wohl–Ziegler) | Initiated by radicals (Br•). | Selective at allylic H; mild conditions. | Alkene + NBS → Allylic bromide |
| Electrophilic | Less common; mostly aromatic systems. | Allylic position attacked by electrophile. | Allyl benzene + Br2 |
Pay attention to questions differentiating which mechanism occurs, as SN1, SN2, SN1', and SN2' often appear side by side in mock tests and boards.
Mechanism of Free Radical Allylic Bromination (Wohl–Ziegler Reaction)
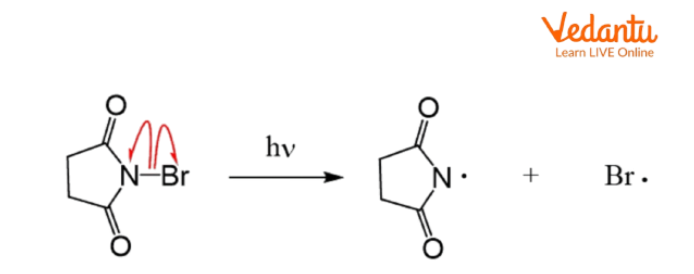
The classical free radical mechanism for allylic substitution is the Wohl–Ziegler bromination, where N-bromosuccinimide (NBS) provides a steady, low concentration of Br2 for selective bromination at the allylic position. This process proceeds via:
- Initiation: Homolytic cleavage of the N–Br bond in NBS forms a Br• radical.
- Propagation: Br• abstracts allylic H, forming an allylic radical stabilized by resonance.
- Allylic radical reacts with Br2 to give allylic bromide.
- NBS maintains low Br2 to prevent direct addition across the double bond.
The resonance effects stabilize the allylic radical or carbocation, explaining faster rates and easier product prediction compared to non-allylic systems.
Essential Reagents and Conditions for Allylic Substitution
Remembering reagents and their effects is a frequent MCQ theme. Here's a compact chart for JEE revision:
| Reagent | Role/Type | Main Condition | Substitution Type |
|---|---|---|---|
| NBS (N-Bromosuccinimide) | Brominating agent | Light/Heat, CCl4 | Free Radical |
| Br2 | Halogen source | With hv, high conc. | Radical/Addition |
| Aq. KOH, CN- | Nucleophiles | Allyl halide, solv. effects | SN1', SN2' |
| H2O (± acid) | Hydrolysis | Mild heat | SN1'/SN2' |
Use memory aids: "NBS needs Light," "Allylic halide, strong nucleophile for SN2'," and "Resonance helps Allyl avoid SN1 traps."
Solved Examples and JEE Practice Questions
Practising key types of questions will secure marks in both Section A and Section B:
- Predict the major product when propene reacts with NBS in CCl4 and light.
- Given: Allyl chloride + aqueous KOH → ? (Mechanism type? Stereochemistry?)
- Which reacts faster in SN2': allyl or n-propyl chloride? (Reason: resonance stabilizes allylic transition state)
- Select which alkene undergoes homolytic allylic substitution most efficiently.
For worked solutions and more exam-style approaches, see Vedantu's SN1 and SN2 Reactions in Organic Chemistry, Nucleophilic Substitution Reaction, and Chemistry PYQs for JEE Main.
Allylic Rearrangement and Industrial Applications
In some allylic substitution reactions, the double bond migrates during substitution (rearrangement)—for example, SN2' attack on an allylic halide produces a new π-bond position. Industrially, allylic bromination (especially via NBS) is used to synthesise intermediates for pharmaceuticals, agrochemicals, and polymers. The selectivity and mildness of these reagents allow chemists to manipulate molecules for large-scale processes, reducing waste and increasing safety.
Summary Table: Exam Tips and Quick Revision
| Quick Tip | JEE Application |
|---|---|
| Allylic = next to C=C | Identify position first in MCQs |
| Resonance-stabilized intermediates | Rapid reactions, expect more SN1'/SN2', radical |
| NBS for radical bromination | Selective substitution, avoid dibromides |
| Compare with acyl/benzylic cases | MCQ traps often test these differences |
| Remember prime notation (') | Means allylic substitution with rearrangement |
- Always draw resonance forms to explain stabilization.
- Practice product prediction for both nucleophilic and free radical pathways.
- Compare allylic substitution with electrophilic, benzylic, and acyl substitutions to avoid confusion in questions.
- Use Vedantu’s chemistry resources for more solved problems on this and other JEE organic reactions.
Stay confident in tackling any Allylic Substitution Reaction appearing in JEE. Key learning—identify, recall mechanism, predict major product, and always check for resonance stabilization. For stepwise mechanism notes and clear product prediction, refer to expert summaries at Vedantu and enhance your Chemistry score.
FAQs on Allylic Substitution Reaction Explained for JEE and Boards
1. What is the allylic substitution reaction?
Allylic substitution reaction is an organic reaction in which a substituent at the allylic position (the carbon atom next to a carbon–carbon double bond) is replaced by another group. This reaction is important because it allows the selective modification of alkenes in both laboratory and industrial synthesis.
Key points:
- Occurs at the allylic carbon (next to a double bond)
- Can proceed via SN1', SN2', or free radical mechanisms
- Commonly seen in questions involving alkenes, allylic halides, or allylic rearrangement
2. Which reagent is commonly used for allylic substitution reactions?
A widely used reagent for allylic substitution is N-bromosuccinimide (NBS) under light or heat, enabling allylic bromination via a free radical pathway. Other reagents include halogens (like Cl2) in the presence of peroxides, and various nucleophiles for SN1'/SN2' mechanisms.
- NBS (with CCl4 and light/heat)
- Halogens (Cl2, Br2) with hv or peroxides
- Nucleophilic reagents (e.g., NaCN, NaOH) for substitution
3. How is allylic substitution different from acyl substitution?
Allylic substitution involves replacing a group at the carbon next to a double bond (alkene), while acyl substitution involves replacement at the carbonyl carbon of acid derivatives (like esters or amides). Key differences include:
- Allylic substitution: Occurs at allylic carbon, common in alkenes
- Acyl substitution: Occurs at acyl (carbonyl) group, common in carboxylic acid derivatives
- Mechanisms, reagents, and intermediates are different
4. What is an example of an allylic rearrangement?
An example of allylic rearrangement is the formation of 1-bromopropene from propene using NBS, where the allylic hydrogen is substituted by a bromine and the double bond may shift.
- Propene + NBS → Allylic bromide + Succinimide
- During the reaction, resonance allows migration of a double bond (rearrangement)
5. Why is the allylic position more reactive in substitution reactions?
The allylic position is more reactive because the intermediates (like carbocations, carbanions, and free radicals) formed during substitution are stabilized by resonance with the double bond.
Key points:
- Resonance leads to delocalized charge
- This increases stability of transition states
- Enables easier loss or gain of substituents
6. What are the types of allylic substitution reactions?
Allylic substitution reactions occur via different mechanisms:
- SN1': Unimolecular nucleophilic substitution with possible rearrangement
- SN2': Bimolecular nucleophilic substitution with double bond migration
- Free radical substitution: Using reagents like NBS and light
- Electrophilic substitution (less common)
7. How can I identify the allylic position in an alkene?
The allylic position is the carbon atom directly adjacent to a carbon-carbon double bond in an alkene.
- Find the C=C double bond
- The carbon(s) bonded to a double-bonded carbon but not part of the double bond are allylic
8. Can allylic substitution occur via both nucleophilic and electrophilic pathways?
Yes, allylic substitution can occur via nucleophilic (SN1', SN2') and electrophilic pathways, but nucleophilic and free radical substitutions are most common in syllabus and exams.
- Nucleophilic: Replacement by nucleophiles (e.g., NaOH, NaCN)
- Electrophilic: Less common, usually in specialized cases
- Free radical: Typical for halogenation with NBS/Br2
9. What are common mistakes when predicting the major product of allylic substitution?
Common mistakes in allylic substitution include choosing the wrong carbon for substitution or ignoring resonance stabilization.
- Missing the correct allylic carbon
- Neglecting possible rearrangement (double bond shift)
- Forgetting resonance structures that stabilize intermediates
10. How do resonance effects stabilize intermediates in allylic substitution?
In allylic substitution, resonance effects allow electrons to delocalize across the allylic system, stabilizing intermediates like carbocations, anions, or radicals.
- Resonance spreads charge over multiple atoms
- This stabilization makes substitution easier and more selective
- Explains the high reactivity of allylic positions in substitution reactions
11. Which reagents are used for free radical allylic bromination?
For free radical allylic bromination, N-bromosuccinimide (NBS) in the presence of light (hv) or heat is the standard reagent. Sometimes, Br2 with peroxides or light is also used. This method selectively replaces an allylic hydrogen with bromine—important for Wohl-Ziegler reaction and related exam questions.
12. How do you distinguish allylic from benzylic substitution reactions?
Both allylic and benzylic substitutions occur at carbons adjacent to a π-system, but:
- Allylic substitution: At carbon next to a C=C double bond in alkenes
- Benzylic substitution: At carbon next to a benzene ring (aromatic system)
































