




Introduction to Aromatisation
Aromatic hydrocarbons or arenes are a particular type of unsaturated hydrocarbon that consists of a parent member, a six-membered carbon ring called benzene or a benzene-like structure. Some of the aromatic hydrocarbons have distinctive aromas. Benzene, and all the larger arenes, have the following characteristics:
1. Aromatic molecules should be cyclic.
2. It should have a planar structure.
3. Aromatic rings should have sp2 hybridised atoms which can form a delocalised system of π electrons.
4. The number of π electrons must be equal to 4n + 2, where n is an integer. (Hückel rule)
Benzene and its derivatives have applications in high-octane gasoline and the production of polymers, insecticides, detergents, dyes, and many miscellaneous chemicals. Hence, they are produced on a large scale. Thus, the conversion of any nonaromatic hydrocarbon structures, especially those found in petroleum, into useful aromatic hydrocarbons is highly significant. This conversion process is called aromatisation. Let us next define aromatisation with an example.
What is Aromatisation?
Aromatisation is a chemical reaction in which a single nonaromatic precursor is converted to an aromatic system. Generally, aromatisation is achieved by dehydrogenation of existing cyclic compounds in the presence of a catalyst and at specific reaction conditions like temperature, pressure, etc. Aromatisation includes the formation of heterocyclic systems or aromatic compounds from precursors like alkane or cycloalkane with six or more carbon atoms under suitable conditions. This process is also called Reforming.
Methods of Aromatisation
1. Oxidative Dehydrogenation: Dehydrogenation is the simplest method of aromatisation of compounds such as cyclohexane, cyclohexene, and cyclohexadiene. Dehydrogenation means the removal of hydrogen to produce unsaturation. Platinum-catalysed dehydrogenations of cyclohexanes and related feedstocks in the petroleum refining industry are the most extensive applications of this reaction.
The dehydrogenation reactions are carried out at temperatures of approximately 300oC with platinum or palladium catalysts. This reaction can also be carried out under milder conditions if a hydrogen acceptor, like maleic acid, is present to remove hydrogen as it is formed. Also, if the reaction is carried out at higher temperatures, the equilibrium will shift towards dehydrogenation because a larger number of molecules causes an increase in entropy. The reaction below explains the aromatisation of hexane.
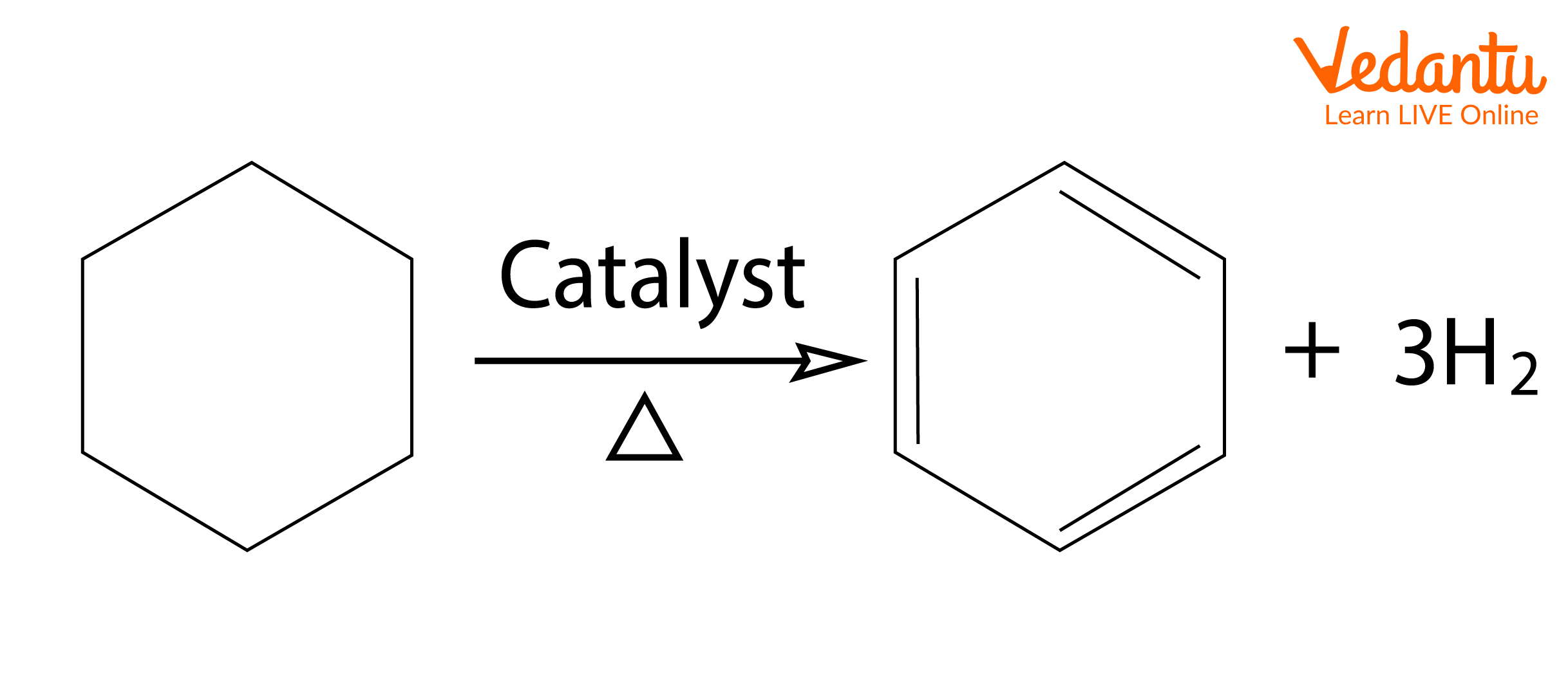
Dehydrogenation of Cyclohexane
2. Dehydration: Non-aromatic rings can be aromatised by dehydration reaction. Dehydration means the removal of water molecules. Here, 2-cyclohexenone oxime is dehydrated to form aniline under acidic conditions.
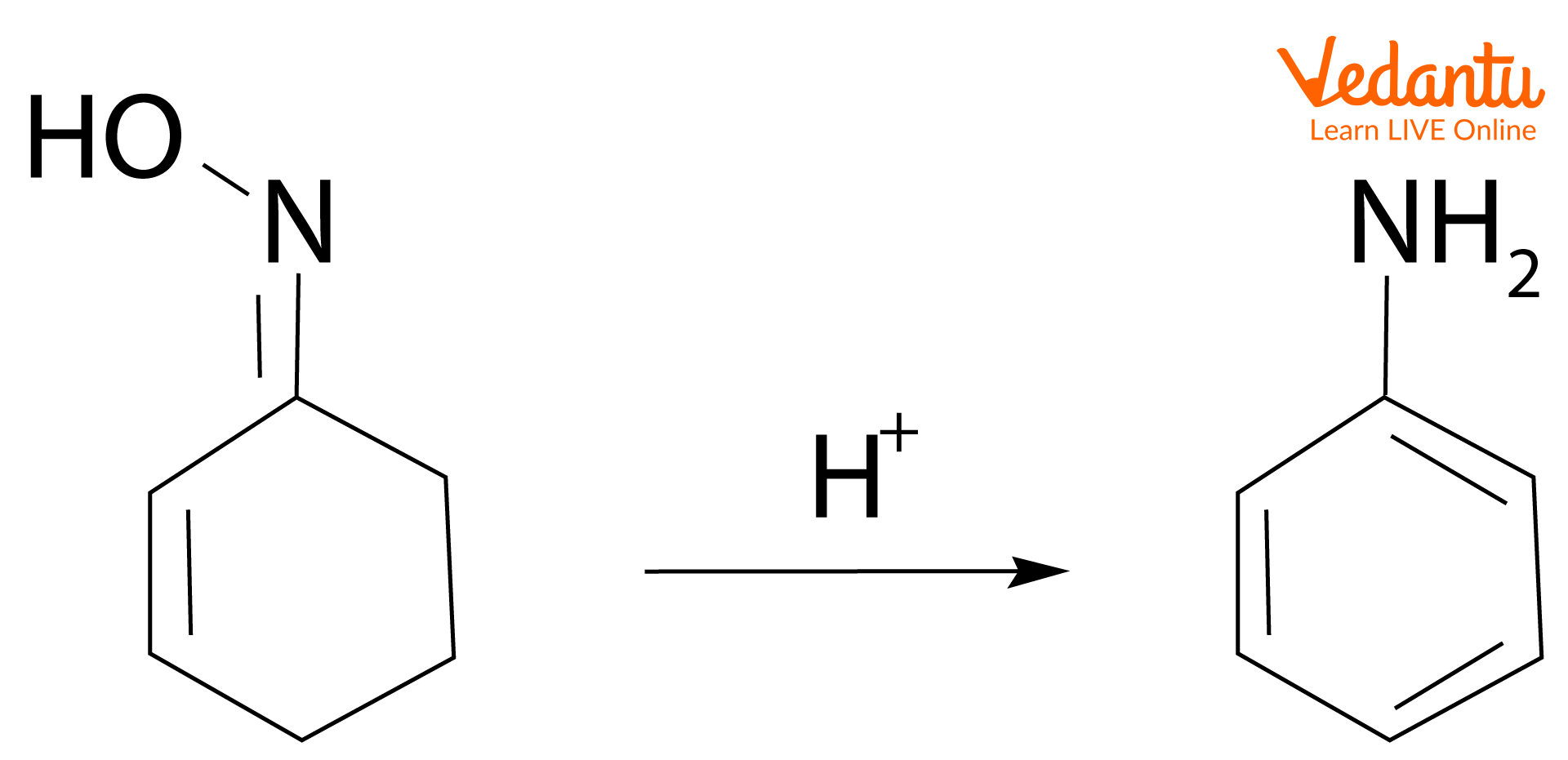
Synthesis of aniline by dehydration
3. Tautomerization:
Tautomers are two molecules with the same molecular formula but have different structures. They are constitutional isomers, which can interconvert in rapid equilibrium. In tautomerization, protons are transferred from one site to another by a series of steps in the presence of a solvent. The isomerization of cyclohexadienones produces the aromatic tautomer phenol. Isomerization of 1,4-naphthalenediol produces a 2:1 mixture with its keto form, 1,4- dioxotetralin at 200oC, and they are in rapid equilibrium.
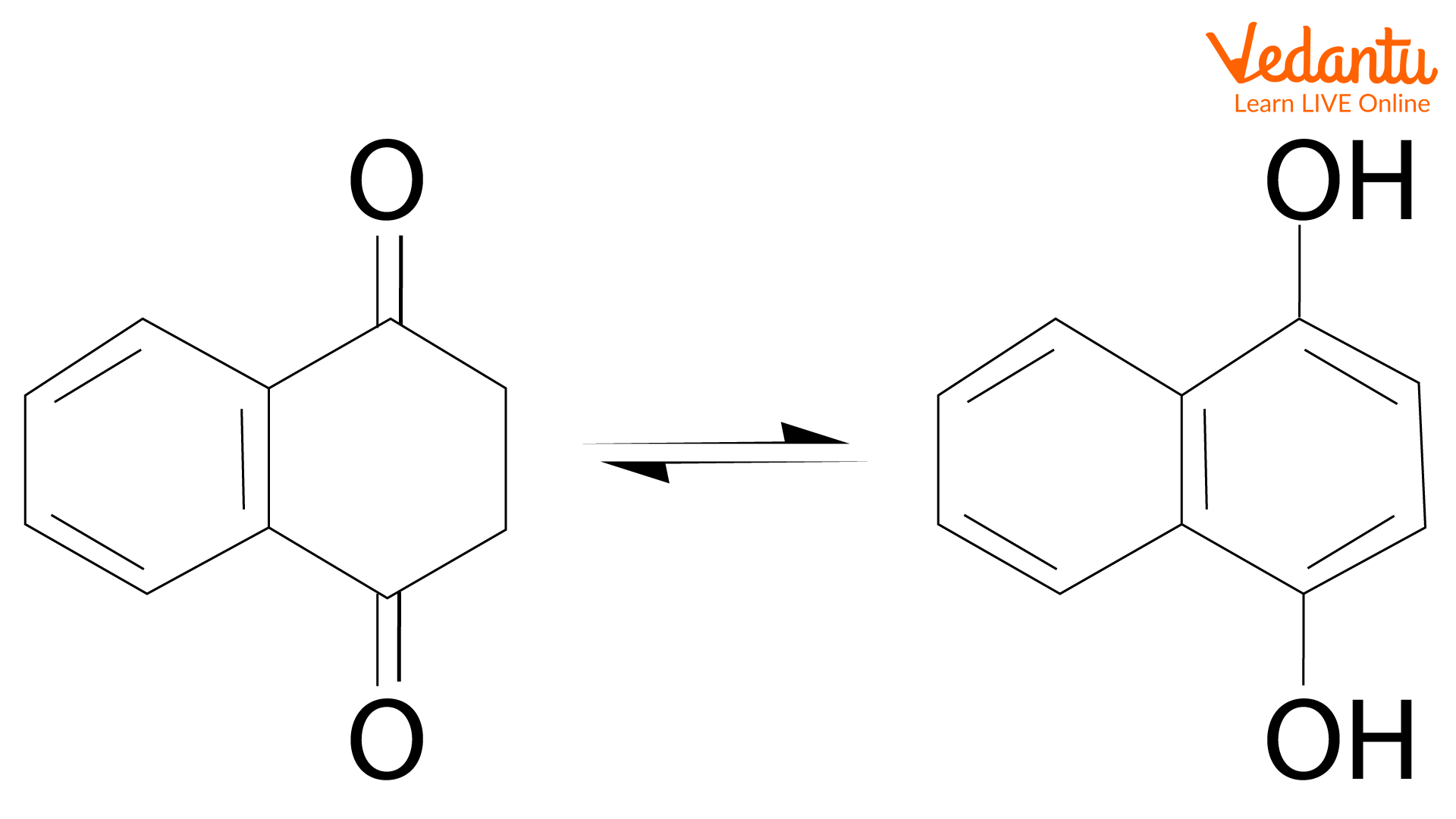
1,4-Dioxotetralin and its aromatised tautomer 1,4-naphthalenediol
Industrial Application
In the petroleum industry, two types of reforming or aromatisation techniques are used to convert low-quality gasoline stocks to improve their combustion characteristics.
Thermal reforming: Convert low-grade naphtha to high octane number molecules by exposing the materials to high temperatures and pressures
Catalytic reforming: Converts low-grade naphthas (mixture of straight-chained and cyclic aliphatic hydrocarbons having from five to six carbon atoms) into high-octane reformate product (aromatic hydrocarbons) by contacting them with a platinum-containing acidic catalyst at high temperatures and pressures. These high octane aromatic hydrocarbons are used for gasoline blending and producing high-value aromatics for petrochemical processing.
For example, the conversion of methylcyclohexane (a naphthene) into toluene (an aromatic) by Dehydrocyclization in the presence of a platinum catalyst.
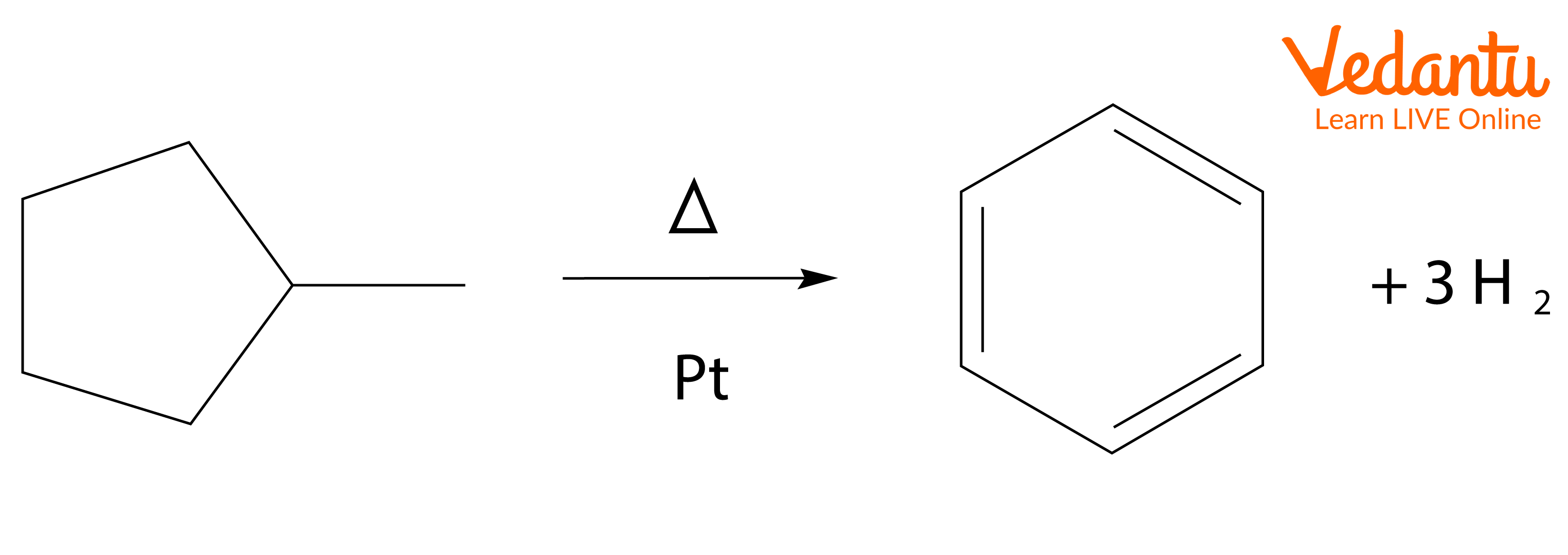
Aromatisation reaction starting with methylcyclopentane
Thus, high octane aromatisation is very important in the petroleum industry.
Application in Biochemical Processes
Aromatase is the enzyme found in the human body that catalyses the conversion of androgens to estrogens and also partially aromatises steroids. Some examples are aromatisation of testosterone to estradiol and androstenedione to estrone. In these aromatisation reactions, oxidation of the C-19 methyl group to formic acid happens, allowing the formation of the aromatic system. These conversions happen in oestrogen tumorigenesis. When estrogen tumorogenesis occurs, it leads to the development of breast cancer and ovarian cancer in postmenopausal women and gynecomastia in men.
The inhibition of this enzyme is required for the treatment of hormone-dependent breast cancer, alterations of ovarian and endometrial function, and treatment of benign disorders such as gynecomastia. Aromatase inhibitors like exemestane, anastrozole, and letrozole prevent the formation of estradiol. This method is more effective than anti-estrogen medications such as tamoxifen. The aromatisation of testosterone to estradiol happens in several tissues of the adult male like adipose, brain, bone, breast, liver, and blood vessels.
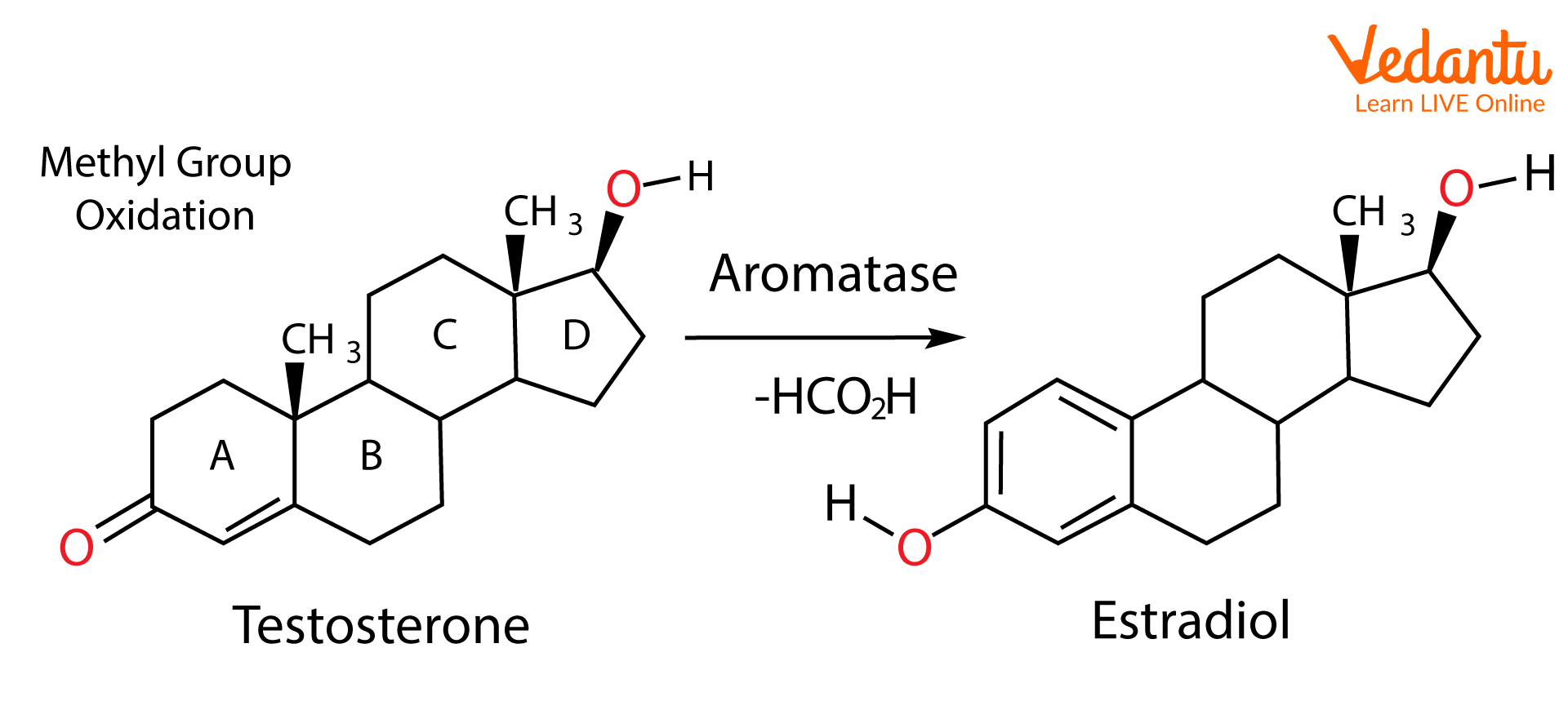
Conversion of testosterone to estradiol by aromatase.
Conclusion
In aromatisation, an aromatic compound is formed from a single non-aromatic precursor. Generally, this type of aromatisation is done by dehydrogenation of existing cyclic compounds so that unsaturation of compounds occurs and they form aromatic compounds. The simplest example of aromatisation is the conversion of cyclohexane into benzene in the presence of a catalyst and under specific reaction conditions. aromatisation mainly includes the formation of heterocyclic systems.
In the petroleum refining industry, aromatisation or reforming is mainly done to convert low octane naphtha fractions into high octane gasoline by either thermal or catalytic reforming. Aromatisation reactions play an important role in our body’s biochemical reactions. When aromatisation occurs as a biochemical reaction, aromatase, a P450 enzyme, converts a nonaromatic ring into an aromatic ring. For example, aromatisation converts androgens into estrogens which contain an aromatic six-carbon ring. Aromatase inhibitors like exemestane, anastrozole, and letrozole prevent the formation of estradiol, which is the cause of breast cancer and ovarian cancer etc.
FAQs on Aromatisation - Using Methods With Applications for JEE
1. What is aromatase and what is the cause of the increase in aromatase?
Aromatase is a naturally occurring enzyme found in the human body. It is present in multiple tissues in the body like the brain, muscles, ovaries, placenta etc. Aromatase is the enzyme which catalyses the aromatisation of testosterone into oestrogen. It also helps to control active amounts of cortisol the body uses to regulate the immune system. If the body has a higher amount of aromatase, more testosterone will be converted into oestrogen during a testosterone spike.
Aromatase is responsible for the development of chronic diseases like cancer and autoimmunity as it aromatises testosterone to estradiol and androstenedione to estrone.
2. What is Octane number? State its importance.
Octane rating is also known as octane number. It measures the quality or performance of gasoline. The higher the octane number, the better the fuel burns within the engine of a vehicle and gives higher performance. The octane rating of a specific gasoline mixture is based on the ratios of two gasoline compounds, which are iso-octane and normal heptane On the octane scale, pure iso-octane has a rating of 100, and n-heptane has a zero rating.
Thus, if you blend 90% iso-octane and 10% n-heptane, it will give an octane rating of 90.

































