




What are Haloarenes?
The hydrocarbons known as haloarenes are those arenes in which one or more hydrogen atoms have been substituted by halogen atoms. Ar-X is the general formula for haloarenes, where Ar is an aryl group and X is a halogen, such as F, Br, Cl, or I. Aromatic compounds with any halogen attached are categorised as haloarenes. Haloalkanes are formed from open-chain hydrocarbons (alkanes), but haloarenes are derived from aromatic hydrocarbons, which is the main distinction between haloalkanes and haloarenes. Haloalkanes and haloarenes are extremely valuable organic compounds. We will discuss the chemical properties of haloarenes in later discussions.
Haloarene
Nucleophilic Substitution Reactions (NSR)
Generally, in normal conditions, haloarenes do not undergo nucleophilic substitution reactions. But in extreme conditions like high temperature and pressure, it undergoes nucleophilic substitution reactions.
The resonant structures of chlorobenzene suggest that the partial double bond properties of haloarenes develop in the C-Cl bond. These factors make it difficult for a nucleophile to split a halogen atom into a halide ion. Haloarene's sp2 hybridised carbon of the C-Cl bond strongly attracts an electron from the halogen. Halogen ion release is consequently challenging. Haloarenes, therefore, react with nucleophiles relatively slowly.
Substitution of –OH Group: Treatment of Chlorobenzene with caustic soda (NaOH) at 623K and 300 atm pressure results in the formation of sodium phenoxide. This further treatment with dilute HCl results in the formation of Phenol.
${{C}_{6}}{{H}_{5}}Cl+2NaOH\xrightarrow[300atm]{623K}{{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}O+NaCl$.
${{C}_{6}}{{H}_{5}}ONa\xrightarrow{dil.HCl}{{C}_{6}}{{H}_{5}}OH+NaCl$
Substitution of –NH2 Group: Treatment of ammonia with chlorobenzene at 200 degrees Celsius and high-pressure results in substitution of halogen with NH2 group, and formation of Aniline.
$2{{C}_{6}}{{H}_{5}}Cl+2N{{H}_{3}}+C{{u}_{2}}O\xrightarrow[\text{Pressure}]{473K}2{{C}_{6}}{{H}_{5}}N{{H}_{3}}+2CuCl+{{H}_{2}}O$
Substitution of –CN Group: Treatment of Chloro-benzene with Cuprous Cyanide (CuCN) at 473K under high pressure in the presence of pyridine or DMF to form Cyanobenzene or Benzonitrile.
${{C}_{6}}{{H}_{5}}Cl+NaCN+CuCN\xrightarrow[\text{Pressure}]{473K}{{C}_{6}}{{H}_{5}}CN+C{{l}^{-}} $
Electrophilic Substitution Reactions
Haloarenes undergo electrophilic substitution reactions with aromatic components. Halobenzene undergoes electrophilic substitution at a slower rate than benzene. Halogen is deactivating as a result of its -I nature. The chlorine's single electron pair engages in resonance with the ring. At ortho and para positions, the electron density rises. In an electrophilic substitution reaction, an electrophile substitutes another electrophile in an organic compound.
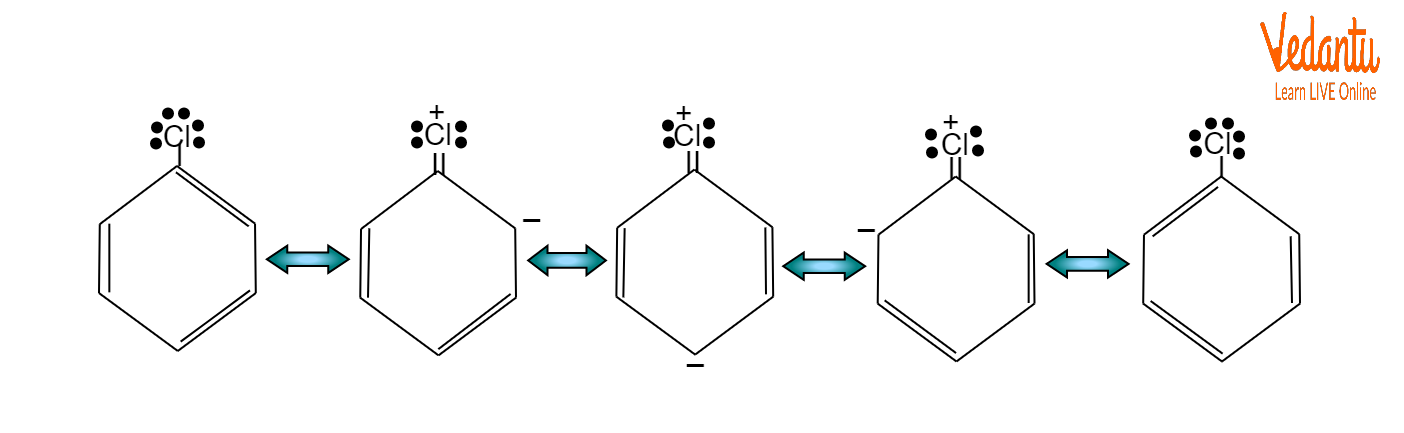
Resonance in Haloarenes
Reactions of Haloarenes
Halogenation
Halogens like bromine are added to the phenyl ring in the presence of aluminium bromide, which acts like a catalyst. Another catalyst that can be used is ferric bromide. The result is the substitution of a bromine atom for an aromatic proton on the phenyl ring.
${{C}_{6}}{{H}_{5}X}\xrightarrow{B{{r}_{2}},AlB{{r}_{3}}}{{C}_{6}}{{H}_{4}}XBr$
Nitration
In nitration of the phenyl ring, concentrated nitric acid and concentrated sulphuric acid react with the benzene ring that puts a nitro group on the phenyl ring.
${{C}_{6}}{{H}_{5}X}\xrightarrow[{{H}_{2}}S{{O}_{4}}]{HN{{O}_{3}}}{{C}_{6}}{{H}_{4}}XN{{O}_{2}}$
Sulphonation
Sulphuric acid is added to the phenyl ring. In this sulphonation of the phenyl ring, water is also formed as a by-product.
${{C}_{6}}{{H}_{5}X}\xrightarrow{{{H}_{2}}S{{O}_{4}}}{{C}_{6}}{{H}_{4}}XS{{O}_{3}}H+{{H}_{2}}O$
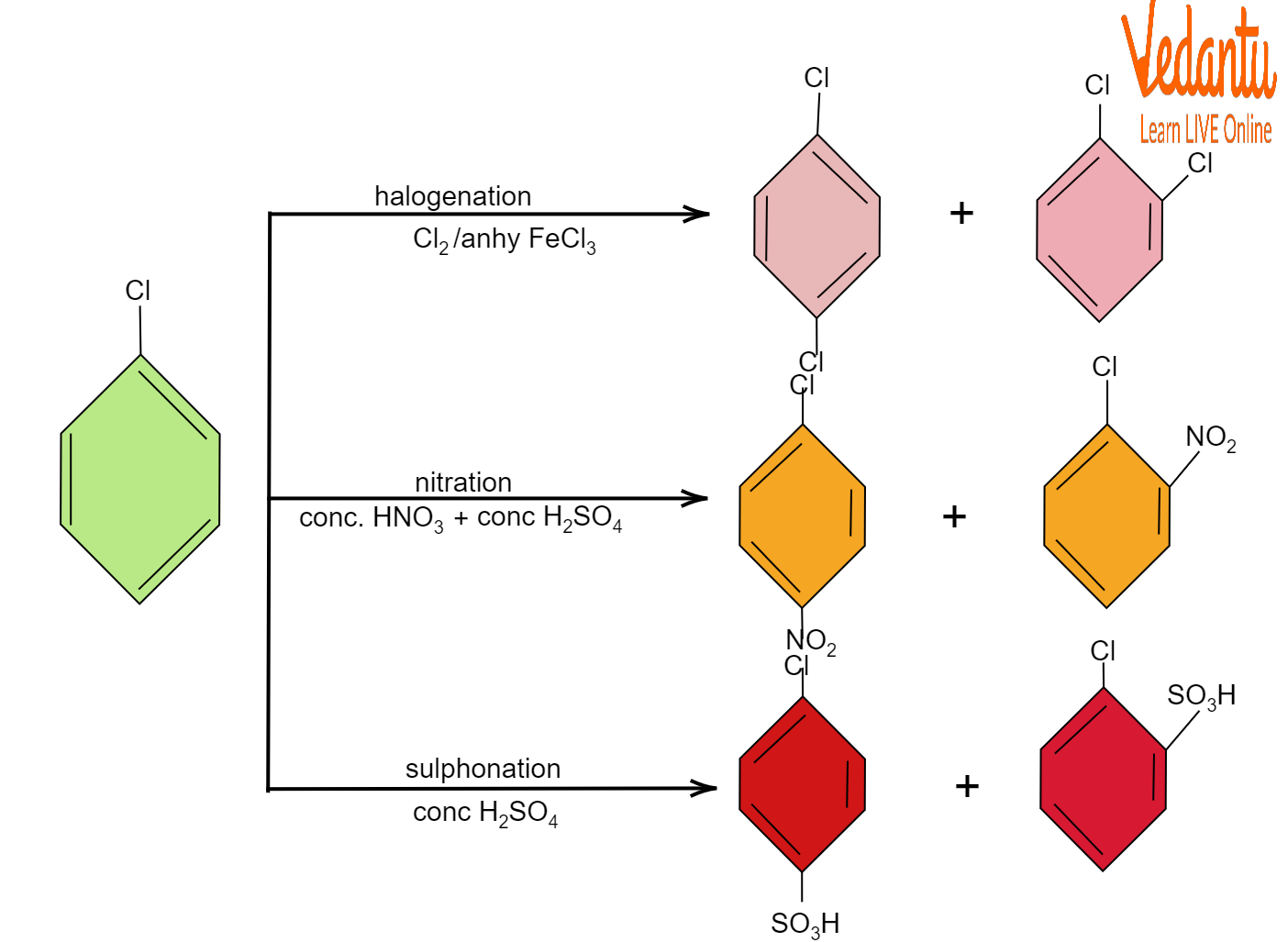
Haloarene Reactions
Halo group is ortho para directing as shown above.
Friedel Crafts Alkylation
To the phenyl ring, an alkyl group is added in the presence of aluminium chloride as a catalyst. The result is substituting an alkyl group (R) (which was on alkyl chloride) for a proton on the aromatic ring.
${{C}_{6}}{{H}_{5}X}\xrightarrow[AlC{{l}_{3}}]{RCl}{{C}_{6}}{{H}_{4}}XR$
Friedel Crafts Acylation
This reaction is very similar to Friedel Crafts Alkylation. An acyl group is added to the phenyl ring in the presence of aluminium chloride as a catalyst. As a result, the acyl group is substituted to the phenyl ring for one of the aromatic protons.
${{C}_{6}}{{H}_{5}X}\xrightarrow[AlC{{l}_{3}}]{ROCl}{{C}_{6}}{{H}_{4}}XOR$
Reactions with Metals
Wurtz fittig Reaction
When heated with sodium in an ether solution, halo arenes react with halo alkanes to produce alkyl benzene. The term Wurtz Fittig Reaction refers to this process.
${{C}_{6}}{{H}_{5}}Cl+2Na+{{C}_{2}}{{H}_{5}}Cl\xrightarrow[\Delta]{Ether}2NaCl+{{C}_{6}}{{H}_{5}}-{{C}_{2}}{{H}_{5}}$
Fittig Reactions
In a dry ether reaction involving haloarenes and sodium metal, two aryl groups combine to form bi-aryl products. This reaction is known as the Fittig reaction.
$2{{C}_{6}}{{H}_{5}}Cl+2Na\xrightarrow[\Delta]{Ether}{{C}_{6}}{{H}_{5}} {{C}_{6}}{{H}_{5}}+2NaCl$
It is one of the Wurtz Fittig Reaction examples.
Physical Properties of Haloarenes
Melting and Boiling Points: All mono-halo benzene's boiling points, which are liquids, follow the order: Iodine is followed by bromine and chlorine. The boiling points of isomeric di-halobenzene are nearly the same. In general, the melting values of ortho and meta isomers are lower than those of para isomers. Due to its symmetry, the p-isomer has a higher melting point resulting in more closed packing in its molecules in the crystal lattice and strong intermolecular attractive force which requires more energy for melting.
Solubility: Haloarenes cannot establish hydrogen bonds with water, hence they are insoluble in water. However, they can dissolve in organic solvents.
Density: All haloarenes are heavier than water and the order for their densities is as follows: Iodo-benzene > Bromo-benzene > Chlorobenzene.
Uses of Haloarenes
The following is a list of some significant applications.
These organic substances are employed as solvents because they can dissolve non-polar substances.
Alkyl and aryl halide compounds are widely employed in medicine.
The drug chloramphenicol, which is used to treat typhoid cases, is such an instance.
Another instance is chloroquine, which is excellent for treating malaria.
DDT, often known as dichlorodiphenyltrichloroethane, is a popular insecticide.
Conclusion
We use hydrocarbons frequently in our daily lives. Because they are used in medicines, these haloalkanes are critical to human survival as well as the survival of all living things. Haloalkanes and haloarenes are precious organic compounds. These are used as solvents, propellants, and for a variety of other industrial applications.
FAQs on Chemical Properties of Haloarenes - JEE Important Topic
1. Why is the Wurtz reaction not suitable for tertiary alkyl halides?
Wurtz's reaction is an organic chemical coupling reaction in which sodium metal reacts with two alkyl halides in the presence of a dry ether solution to form a higher alkane as well as a compound containing sodium and the halogen. Because of steric hindrance, nucleophilic attack on a tertiary alkyl halide is extremely slow. That is why the Wurtz reaction fails with tertiary alkyl halides.
2. How does the catalyst aluminium chloride function in Friedel Crafts Acylation and Friedel Crafts Alkylation?
The mechanism of Friedel Crafts Acylation is similar to that of Friedel Crafts Alkylation. In both reactions, the catalyst aluminium chloride functions as a Lewis acid and accepts a pair of electrons. The chlorine from acyl chloride donates a pair of electrons and aluminium from aluminium chloride accepts that electron pair.
3. Why is the NO2 group only effective in the Ortho and Para positions but not in the Meta?
The NO2 group is only effective in the Ortho and Para positions but not in the Meta because of the maximum effect of NO2 at otho and para positions because NO2 is from the electron withdrawing group which pulls the benzene ring towards itself. This pulling of electrons causes a decrease in electron density at ortho and para position positions of the benzene ring while the meta position of the benzene ring remains unaffected by this process of pulling of electrons from benzene towards the NO2 group.

































