




Introduction to Chiral Compounds
The word chiral originates from an ancient Greek word ‘cheir’ meaning hand. A compound or a molecule is said to be chiral when they have a non-superimposable mirror image. This means the compound cannot be superimposed on its mirror image; that is, they do not overlap one another. A chiral compound is capable of existing in two configurations, that is as two stereoisomers. These forms are non-superimposable mirror images of each other and are called enantiomers.
Chirality Definition along with Examples
Chirality is the geometric property of chiral molecules. Chiral molecules lack symmetry elements. They do not have a plane or centre of symmetry. A plane of symmetry or centre of symmetry is an imaginary line or a plane that divides the molecule into two equal halves such that they are mirror images of each other. The existence of chirality in a molecule is mainly due to the presence of a chiral centre.
Chiral centre, also known as chirality centre or asymmetric centre of an atom, is an atom in a molecule that is attached to four different chemical species or functional groups in a spatial arrangement such that it can exist as two enantiomers.
Example 1: Human hand is the best example of chirality. The left hand is a non-superimposable mirror image of the right hand and vice versa. When the right hand is placed on the left hand or the other way round, they do not superimpose. It is impossible for them to coincide with one another irrespective of their orientation.
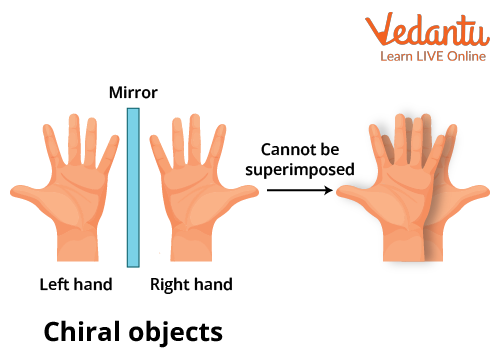
Hand as an Example of Chirality
Example 2: Butan-2-ol and its mirror image are non-superimposable. Therefore it is a chiral compound.
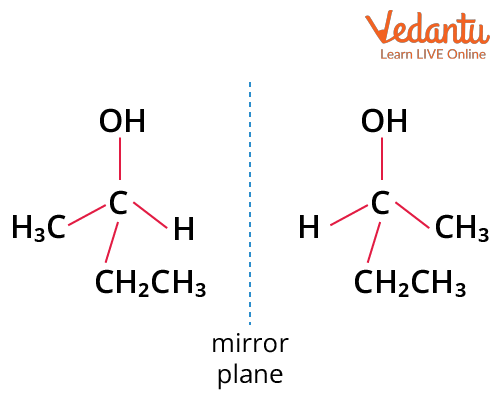
Butan-2-ol Showing Chirality
Chiral Carbon
Now that we understand chirality, we can answer the question: what is a chiral carbon? Chiral carbon, also known as asymmetric carbon or stereogenic carbon, is a carbon atom attached to 4 different types of atoms or functional groups that are placed at four corners of a tetrahedron in space.
Example: Carbon number 2 of 2-butanol is a chiral carbon because it is attached to 4 different substituent groups that are: a hydrogen (-H) atom, ethyl (-CH2CH3) group, a hydroxyl (-OH), and a methyl (-CH3) group.
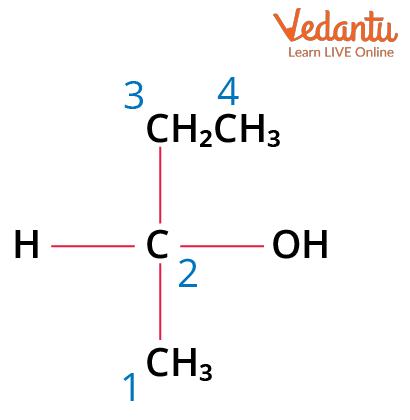
2-butanol with a Chiral Carbon
Difference between Chiral and Achiral Molecules
Optical Activity
Optical activity is defined as the ability of a chiral compound to rotate the plane of plane-polarized light passed through it. Based on the direction of rotation of the plane polarized light, a chiral molecule can be classified as dextrorotatory and levorotatory.
If the plane polarized light is rotated by an enantiomer in a clockwise direction, it is dextrorotatory (dexter meaning right in Latin). It is denoted by the prefix d- or (+)- .
If the plane polarized light is rotated by an enantiomer in a counterclockwise direction, it is levorotatory (laevus meaning left in Latin). It is denoted by the prefix l- or (-)- .
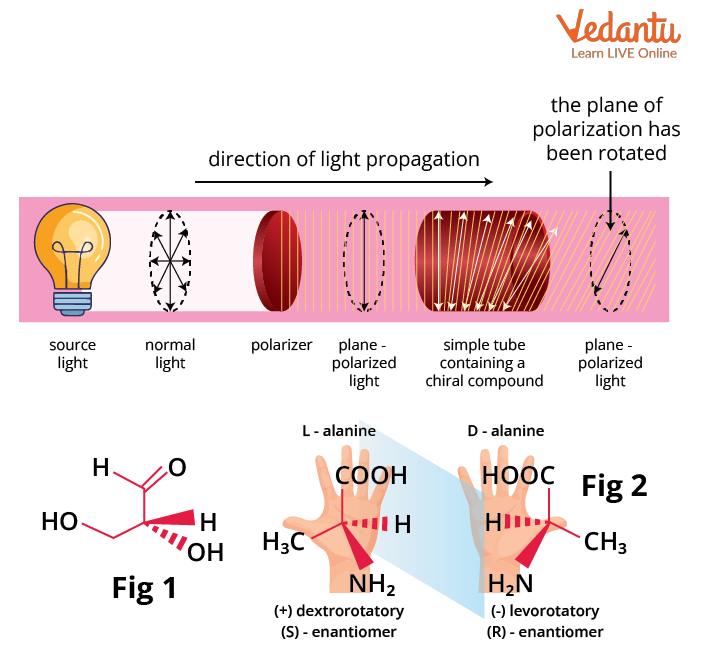
Examples of Dextrorotatory and Levorotatory
Enantiomers
Enantiomers are a pair of molecules or isomers that are non-superimposable mirror images of one another. These two compounds have the same connectivity, meaning the same chemical bonding but different or opposing three-dimensional orientation. They can be easily differentiated from one another. The best way to understand this is using a human hand. The left hand and right hand are like enantiomers since they are mirror images of one another and are not superimposable. The right hand can be easily differentiated from the left hand.
For a molecule to be an enantiomer, the first and foremost criterion is that it should have a chiral centre or a chiral carbon. In addition, it should be devoid of the plane of symmetry.
Example: dextro lactic acid and levo lactic acid
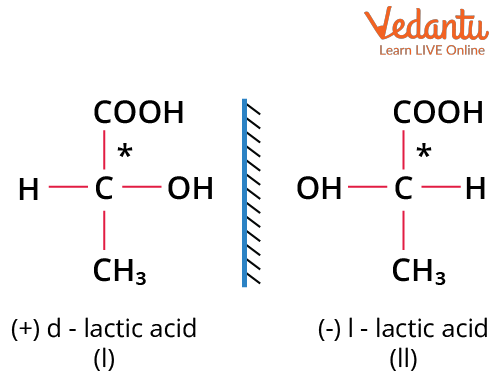
d-lactic Acid and l-lactic Acid as Examples of Enantiomers
Coming to the major difference between a chiral and enantiomers, chiral explains what a single atom looks like. There is no comparison involved, while enantiomers speak of the molecule itself and not a specific atom. It compares and describes how the two molecules can be related. It is a subset of chiral compounds.
Conclusion
Chiral compounds are those which have non-superimposable mirror images. They exist in the form of 2 stereoisomers referred to as enantiomers. They lack a plane of symmetry. The chirality is the geometric property of these chiral compounds. Chirality can be classified as dextrorotatory or levorotatory based on its optical activity. Chiral carbon is the carbon atom attached to four different functional groups. Enantiomers are a pair of molecules or isomers that are non-superimposable mirror images of one another.
FAQs on Chiral Centre and Enantiomers - JEE Important Topic
1. What are meso compounds?
Meso compounds, also known as meso isomers are stereoisomers. They are achiral compounds having two or more chiral centres or stereocentres. They have an internal mirror plane or a plane of symmetry that divides the molecule into equal halves such that each half is a mirror image of another. This internal mirror plane makes them optically inactive even after having two or more stereocentres. Thus, meso compounds are diastereomers, meaning they are stereoisomers that are not enantiomers.
Example: 2,3-butanediol
2. What is a racemic mixture?
A racemic mixture, also known as a racemate is a solution containing equal quantities of each member of a pair of enantiomers. They are optically inactive. This is because each enantiomer of the pair rotates the plane of plane-polarized light in a certain direction through a characteristic angle, but this is exactly opposite to each other and therefore cancels one another making the net rotation zero. In other words, the amount of dextrorotatory molecules is the same as the amount of levorotatory molecules.
Racemization is the process of conversion of an optically active substance into a racemic mixture, making them optically inactive. Many chiral drugs are synthesized as racemates.
Example: Ibuprofen
























