




What are Diastereomers?
Stereoisomers are the compounds that have the same composition and order of covalent bonds, but the main difference is in relative positions of their atoms or groups in space. This type of isomerism is called stereoisomerism; and it is of two types, geometrical and optical isomerism. Diastereomers are stereoisomers which are non-superimposable molecules that are not mirror images of each other. So, they are non-mirror images, non-identical stereoisomers. A stereocenter is an atom in a molecule that has four different atoms or groups of atoms attached to it. Generally, a carbon atom will be a stereocenter. Diastereomers exist when two or more stereoisomers of a compound have different configurations at one or more of equivalent stereocenters and they should not be mirror images of each other.
Diastereomers Definition
Stereoisomers are compounds with the same chemical formula and same atom connectivity. However, they have different 3D shapes and are not superimposable in space. Non-superimposable means they cannot look the same when the molecule is rotated in different directions. Diastereomers are stereoisomers which have at least two stereocenters with different configurations at some of the stereocenters but the identical configuration at others.
They are not mirror images of one another. But enantiomers are chiral molecules that are mirror images and are not superimposable. Enantiomers are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Diastereomers are the stereoisomers which are not mirror images of one another and that are not superimposable. The example of diastereomers is cis-2-butene and the trans-2-butene.
If two diastereomers differ from each other at only one stereocenter they are called epimers. Diastereomers have different physical properties and sometimes different chemical reactivity also.
Diastereomers can also occur at a double bond. Here the cis vs trans relative positions of substituents give two non-superposable isomers. Diastereoselectivity means preference to form one or more than one diastereomer over the other in an organic reaction.
Diastereomers Example
Consider aldopentose, four diastereomers are possible for this compound. These four diastereomers have different arrangements of molecules in space and are on. They all are non-mirror images and non-superimposable. Diastereomers occur in a compound when two or more stereoisomers have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other. When two diastereomers vary from each other at only one stereocenter, they are called epimers. Here we can see in the diastereomer, each stereocenter gives rise to two different configurations.
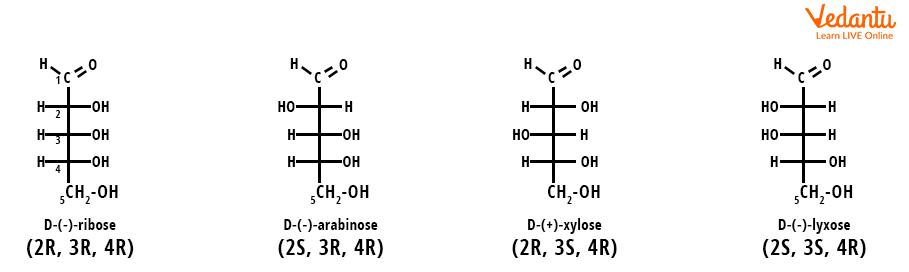
Structure of Aldopentose, A Diastereomer
The following compound gives a cis/trans pair of cyclohexane 1-4 dimethanol, which is a pair of diastereomers.
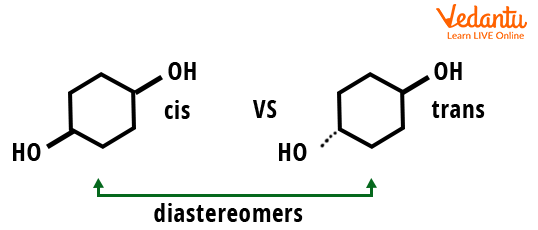
Structure of a Diastereomer Cyclohexane 1-4 Dimethanol.
When a diastereomer has achiral carbon, the configuration is called Threo and erythro configurations. A chiral molecule or ion cannot be superposed on its mirror image by any combination of rotations, translations, and due to conformational changes. In Saccharides, the erythro isomer has two identical substituents on the same side and the threo isomer has them on opposite sides. However, in a zig-zag chain, the erythro isomer has two identical substituents on different sides of the plane.

Structure of a Diastereomer with Threo and Erythro Configurations
Properties of Diastereomers
Other than geometrical isomers, diastereomers may or may not be optically active.
Diastereomers have different physical properties like melting point, boiling point, refractive index, dielectric constant, specific rotation, density, etc.
Since physical properties are different, diastereomers can be separated by fractional crystallization, chromatography, fractional distillation, etc.
Diastereomers are non-mirror images and non-superimposable.
These isomers have different chemical reactivity
Diastereomer are characterized with same structural and chemical formula
The solubility of Diastereomers is different.
Arrangement of atoms in space is different for diastereomers, meaning different 3D shapes.
Diastereomerism can happen at a double bond.
Conclusion
Diastereomers are non-mirror image, non-superimposable and optical active compounds. They have identical structural, chemical and molecular formulas. The arrangement of atoms of their molecules in space is different. Diastereomers have different configurations (R and S) at two or more chiral centers or stereocenters but not all. They have different melting points, boiling points and densities. They can be separated by fractional distillation and chromatographic techniques.
FAQs on Diastereomers - JEE Important Topic
1. State the difference between an enantiomer and a diastereomer.
Enantiomers and diastereomers are stereoisomers. Enantiomers have mirror images and non-superimposable chiral centers. Diastereomers contain non-mirror image, non-superimposable chiral centers. double-bond isomers or cis-trans isomers will always be diastereomers. The enantiomer of a molecule will always have an opposite R/S configuration. Diastereomers compounds arise when at least two molecules share at least one chiral center with identical $\left(\dfrac{R}{S}\right)$ configuration. For example, there are two forms of glucose (the most common sugar molecule), D-glucose and L-glucose, they are enantiomers.
2. Are diastereomers optically active?
Optically active compounds are exactly chiral compounds. Since they do not have mirror symmetry, if we take a mirror image of such a chiral compound, we will get another compound. They are called diastereomers. Stereoisomers that aren't mirror images of each other are called diastereomers. As they lack mirror symmetry, they are optically active. Substances that can rotate plane-polarized light are said to be optically active. Those that rotate the plane clockwise are said to be dextrorotatory. Those that rotate the plane counterclockwise are called levorotatory.

































