




Stepwise Oxymercuration Demercuration Mechanism with Markovnikov Addition
The Oxymercuration Demercuration reaction is a gold standard for adding water to alkenes in organic chemistry, allowing rapid, Markovnikov-selective conversion of double bonds to alcohols without carbocation rearrangement. This makes it crucial for JEE Main aspirants, especially when accurate product prediction and mechanism drawing are needed for numericals and MCQs. Mastering oxymercuration-demercuration is also key to understanding the contrast between acid-catalysed hydration and hydroboration-oxidation, all vital concepts for the hydrocarbon chapters.
Oxymercuration-demercuration is an electrophilic addition hydration reaction typically performed in two steps, using mercury(II) acetate [Hg(OAc)2] and water, followed by reduction with sodium borohydride (NaBH4). The process ensures Markovnikov addition and formation of alcohols without rearrangement. Variants like alkoxymercuration (using alcohol instead of water as the nucleophile) expand its utility in synthetic organic chemistry. Applications include safe hydration of sensitive or hindered alkenes where classic acid-catalysed routes would lead to by-products or incorrect regiochemistry.
Oxymercuration Demercuration: Stepwise Mechanism
Oxymercuration demercuration mechanism proceeds in two main stages. Below is a structured breakdown for JEE:
- Electrophilic attack: Alkene reacts with Hg(OAc)2 to form a three-membered cyclic mercurinium ion intermediate.
- Nucleophilic addition: Water (or alcohol) attacks the more substituted carbon, opening the ring (anti addition).
- Deprotonation: Removal of a proton from the oxonium ion to form the organomercury alcohol intermediate.
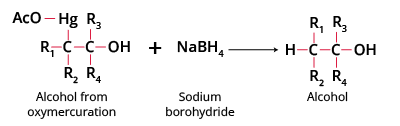
- Reduction: NaBH4 replaces the HgOAc group with hydrogen, yielding the final alcohol product.
Step1 Attack of electrophile forms the mercurinium ion, preventing free carbocation intermediates and thus avoiding rearrangement.

Step2 cleavage of the C-Hg bond occurs when water attacks from the side opposite mercury (anti addition).
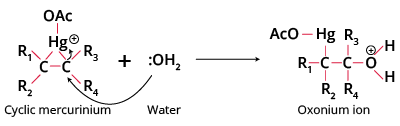
Step3 deprotonation of oxonium ion yields an organomercury alcohol; NaBH4 then reduces this to an alcohol.

Step 4 Formation of alcohol completes oxymercuration demercuration, with NaBH4 replacing Hg by hydrogen. This step finalizes the Markovnikov alcohol product crucial for JEE mechanisms.
Markovnikov vs Anti-Markovnikov: Which Addition Occurs?
Markovnikov addition occurs in oxymercuration-demercuration because the nucleophile adds to the more substituted carbon. This is opposite to hydroboration-oxidation, which yields anti-Markovnikov products. Remember, unlike acid-catalysed hydration, there is no rearrangement in this reaction.
| Feature | Oxymercuration Demercuration | Hydroboration-Oxidation |
|---|---|---|
| Regioselectivity | Markovnikov | Anti-Markovnikov |
| Rearrangement possible? | No | No |
| Stereochemistry | Anti addition (not stereospecific overall) | Syn addition |
For a broader view of Markovnikov and anti-Markovnikov rules, refer to Addition of HX to Alkene and Hydrocarbons.
Roles of Key Reagents in Oxymercuration Demercuration
- Hg(OAc)2: Electrophile; forms mercurinium ion with alkene.
- H2O: Nucleophile; attacks the more substituted carbon for alcohol formation.
- NaBH4: Reducing agent; replaces organomercury group with hydrogen in demercuration.
Understanding roles is crucial for scoring in JEE mechanism questions—especially differentiating between this reaction and classic hydration with H2SO4 as catalyst.
Stereochemistry of Oxymercuration Demercuration: Syn or Anti?
The initial addition across the double bond is anti due to nucleophilic attack from the side opposite to mercury, but the final reaction is non-stereospecific overall because the reduction step with NaBH4 randomizes configuration. Hence, for JEE, expect racemic mixtures or retention/loss of stereochemistry depending on substrate structure.
See also SN1 and SN2 Reactions and Optical Isomerism for nuanced stereochemical outcomes.
Applications: Variations and Alkyne Oxymercuration
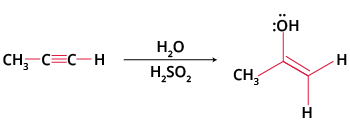
This reaction works with alcohols (alkoxymercuration) for ether synthesis or with alkynes, leading to ketone formation after tautomerisation. For example, oxymercuration of alkynes uses HgSO4/H2SO4 in water, producing Markovnikov ketones, an important transformation for JEE organic synthesis chapters.
Common Mistakes and Top JEE Tips
- Do not expect rearrangement in oxymercuration-demercuration—organomercury intermediates prevent carbocation shifts.
- Distinguish from acid-catalysed hydration, which may rearrange—key for product prediction in JEE.
- Remember Markovnikov product, not anti-Markovnikov—do not confuse with hydroboration-oxidation.
- NaBH4 step is essential—without it, mercury remains in the molecule.
- For alkynes, expect ketones as final products (not alcohols) due to tautomerisation.
- Stereochemistry is generally not controlled in final alcohol product—be careful if asked for configuration in MCQs.
- For ethers, use alcohol (ROH) instead of water in Step 2—watch the nucleophile.
- Refer to Rearrangement of Carbocation for in-depth reasoning on rearrangement avoidance.
Practice Questions: Oxymercuration Demercuration in JEE Exams
- Predict the major product: CH2=CH–CH2–CH3 + Hg(OAc)2/H2O, then NaBH4.
- Identify the key difference in mechanism between acid-catalysed hydration and oxymercuration.
- For 3-methyl-1-butene, draw the complete stepwise mechanism with appropriate curved arrows for oxymercuration-demercuration.
- Choose: Oxymercuration of 1-methylcyclohexene yields alcohol at which position? (a) primary (b) secondary (c) tertiary
- If 2,3-dimethyl-2-butene undergoes oxymercuration-demercuration, what is the stereochemistry of the product?
Solutions:
- Major product: 2-butanol (Markovnikov addition, –OH at C2).
- Oxymercuration proceeds via mercurinium ion, no carbocation forms, hence no rearrangement.
- See steps: mercurinium ion formation (electrophilic addition), anti attack of H2O, deprotonation, NaBH4 reduction to alcohol.
- (c) Tertiary carbon; Markovnikov orientation.
- Product will be a meso-compound or racemic mixture; stereochemistry is not fixed due to reduction.
For quick revision, review Organic Compounds Containing Oxygen and test your knowledge using Vedantu's curated practice sets. Understanding Oxymercuration Demercuration not only improves mechanism writing but sharpens your ability to tackle high-weightage JEE organic chemistry sections efficiently.
This guide aligns strictly with JEE Main and NCERT points. For more conceptual clarity, explore detailed problem sets at Vedantu, a trusted source for competitive exam preparation.
FAQs on Oxymercuration Demercuration: Definition, Mechanism, and Applications
1. What is oxymercuration-demercuration and why is it important?
Oxymercuration-demercuration is a two-step organic reaction used to convert alkenes into alcohols using Markovnikov addition without carbocation rearrangement. It is important for JEE and NEET exams because it allows students to predict the correct product of alkene hydration with high reliability.
Key points:
- Adds water across double bonds in alkenes (alkene hydration reaction).
- Uses reagents: Hg(OAc)2/H2O (step 1), NaBH4 (step 2).
- Provides Markovnikov addition (–OH to more substituted carbon).
- Prevents carbocation rearrangement, leading to predictable products.
2. What is the mechanism of oxymercuration-demercuration?
The mechanism of oxymercuration-demercuration involves two distinct steps: oxymercuration (addition of mercuric acetate and water) and demercuration (reduction to replace mercury with hydrogen).
Stepwise process:
- Oxymercuration: The alkene reacts with Hg(OAc)2 to form a three-membered mercurinium ion intermediate.
- Water attacks the more substituted carbon, opening the ring and forming an organomercurial alcohol.
- Demercuration: Treatment with NaBH4 replaces mercury with hydrogen, yielding the alcohol.
3. Does oxymercuration-demercuration follow Markovnikov or anti-Markovnikov rule?
Oxymercuration-demercuration follows the Markovnikov rule, meaning the –OH group attaches to the more substituted carbon of the double bond.
Key points:
- Markovnikov addition is observed because water attacks the more substituted carbon of the mercurinium ion.
- This is in contrast to hydroboration-oxidation, which gives anti-Markovnikov products.
- No carbocation rearrangement occurs during the process.
4. Is oxymercuration-demercuration syn or anti addition?
Oxymercuration-demercuration is not stereospecific; the addition can be either syn or anti.
Key details:
- Water attacks from either side of the mercurinium ion intermediate.
- The resulting alcohol can show a mixture of syn and anti addition products.
- This distinguishes it from purely syn (hydroboration) or purely anti (bromination) additions.
5. What is the role of Hg(OAc)2, H2O, and NaBH4 in oxymercuration-demercuration?
Each reagent in oxymercuration-demercuration has a distinct function:
- Hg(OAc)2: Activates the alkene by forming a mercurinium ion intermediate.
- H2O: Acts as a nucleophile, attacking the more substituted carbon and adding –OH (hydration).
- NaBH4: Reduces the organomercurial intermediate, replacing the mercury with hydrogen (demercuration), yielding a pure alcohol.
6. Will oxymercuration-demercuration cause carbocation rearrangement?
Oxymercuration-demercuration does not cause carbocation rearrangement.
Explanation:
- It proceeds via a three-membered mercurinium ion, not a free carbocation.
- This prevents hydride or alkyl shifts, ensuring product predictability.
- Students can confidently assign the correct regiochemistry in their answers.
7. What is the difference between acid-catalyzed hydration and oxymercuration-demercuration of alkenes?
Oxymercuration-demercuration differs from acid-catalyzed hydration mainly in mechanism and product outcome.
Main differences:
- Oxymercuration-demercuration: No carbocation forms, no rearrangement, uses Hg(OAc)2, H2O, NaBH4.
- Acid-catalyzed hydration: Involves carbocation intermediates, which may rearrange, uses H2SO4/H2O.
- Both yield Markovnikov products, but only oxymercuration-demercuration avoids rearrangement.
8. Can oxymercuration-demercuration be used for alkynes?
Yes, oxymercuration-demercuration can be adapted for alkynes to form ketones.
Key points:
- Treatment of a terminal alkyne with HgSO4 and H2SO4 yields a ketone via enol formation and tautomerisation.
- This method is commonly used in laboratory synthesis for methyl ketones.
9. Why is NaBH4 added after the first step and can it be skipped?
NaBH4 is essential in the second (demercuration) step to replace the mercury group with hydrogen.
Key details:
- Without NaBH4, the intermediate remains as an organomercurial compound, not the desired alcohol.
- Skipping this step gives incomplete reaction and the product is not an alcohol.
- NaBH4 ensures a safe, mercury-free alcohol is obtained.
10. How to distinguish products formed from acid-catalyzed hydration and oxymercuration-demercuration in exams?
Products can be distinguished based on carbocation rearrangement:
- Oxymercuration-demercuration: Always gives the direct Markovnikov alcohol, no rearrangement, even with complex alkenes.
- Acid-catalyzed hydration: May show rearrangement, leading to different alcohols (check for hydride/methyl shifts in skeleton).
- Examiners may test this using 2° or 3° carbons – predict rearrangement only for acid-catalyzed mechanisms.
11. What are common mistakes to avoid when using oxymercuration-demercuration in exams?
To maximize marks, avoid these errors:
- Predicting rearrangement – remember, this reaction avoids it.
- Confusing it with hydroboration (which is anti-Markovnikov and syn addition).
- Forgetting to mention both steps: oxymercuration and demercuration are required for the final product.
- Assuming the reaction is stereospecific – it is actually not.
- Mixing up the roles of Hg(OAc)2 and NaBH4.
12. What does Hg(OAc)2, H2O and NaBH4 do in oxymercuration-demercuration reaction?
In oxymercuration-demercuration reaction:
- Hg(OAc)2 – reacts with the alkene to form a mercurinium intermediate.
- H2O – adds across the double bond (as –OH group) following Markovnikov rule.
- NaBH4 – reduces and removes mercury, yielding a stable alcohol.























