




JEE main 2026: Introduction to Benzaldehyde
Benzaldehyde, an important aromatic compound, is widely studied in organic chemistry and is an important topic for JEE Main 2026. It is commonly prepared through methods like oxidation of toluene, hydrolysis of benzal chloride, and other laboratory techniques. Understanding its preparation, properties, and reactions is essential for mastering key organic chemistry concepts.
Benzaldehyde is an aromatic aldehyde with the molecular formula C6H5CHO and is also known as the oil of bitter almonds. It is a major component of bitter almonds in the form of its glucoside named amygdalin (C20H27O11N). On boiling with dilute acids, amygdalin will hydrolyze into benzaldehyde, glucose, and hydrogen cyanide. Molecular weight of benzaldehyde is 106.12 $\dfrac{g}{mol}$. It was first extracted by a French pharmacist known as Martres in 1803.
JEE Main Chemistry Syllabus JEE Main Chemistry Revision Notes JEE Main Chemistry Important Questions JEE Main Chemistry Difference Between JEE Main Chemistry Question Papers
Structure of Benzaldehyde
Benzaldehyde structure contains a simple aldehydic group attached to a benzene ring as follows.
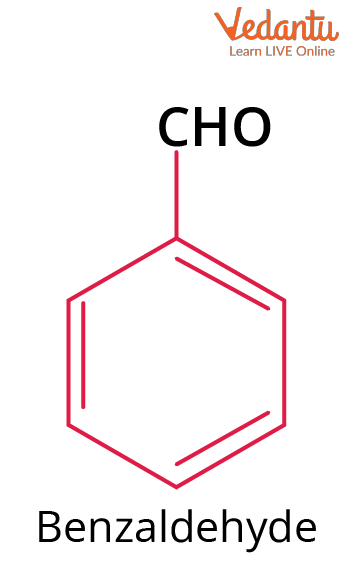
Chemical Structure of Benzaldehyde
Methods of Preparation of Benzaldehyde
Laboratory Method
Benzaldehyde is prepared in the laboratory by boiling benzoyl chloride (C6H5CH2Cl) with copper nitrate (Cu(NO3)2) or lead nitrate (Pb(NO3)2) in the presence of CO2.
$\begin{align} &\underset{\text { Benzoyl chloride} }{2 \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{Cl}}+\underset{\underset{\mathrm{Pb}(\mathrm{NO_3})_2}{or}}{\mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2}} \underset{CO_2}{\xrightarrow{Heat}}\underset{Benzaldehyde}{2\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}}+\mathrm{CuCl}{ }_{2}+2 \mathrm{HNO}_{2} \end{align}$
By Hydrolysis of Benzal Chloride
Hydrolysis of benzal chloride produces benzaldehyde where hydrolysis is done by aqueous caustic soda or milk of lime.
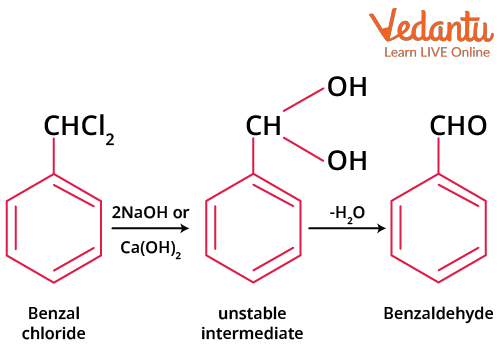
Hydrolysis of Benzal Chloride
By Rosenmund Reaction
This reaction is also called catalytic reduction of benzoyl chloride; in this process, hydrogen is passed through the solution of benzoyl chloride in xylene till the evolution of HCl stops in the solution. This is done in the presence of a palladium catalyst poisoned by BaSO4.
$\underset{\text{Benzoyl chloride}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COCl}}+\mathrm{H}_{2} \underset{\text { (Boiling xylene) }}{\xrightarrow{\mathrm{PdBaSO}_{4}, \mathrm{~S}}} \underset{\text{Benzaldehyde}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}}+\mathrm{HCl}$
In order to prevent the further reduction of aldehyde (benzaldehyde) to respective alcohol, the catalyst is poisoned with a small amount of sulphur or quinoline.
Benzoyl chloride can also be reduced to benzaldehyde by treating it with Lithium tri-tert-butoxyaluminum hydride LiAlH[OC(CH3)3]3 at -780C as follows:
$\underset{\text{Benzoyl chloride}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COCl}}+\mathrm{H}_{2} \underset{\text { (Boiling xylene) }}{\xrightarrow{LiAlH[OC(CH_3)_3]_3~\text{di ethyl ether}, -78^{\circ}C}} \underset{\text{Benzaldehyde}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}}$
Dry distillation of a mixture of calcium benzoate and calcium formate will form benzaldehyde as the main product. The other possible products of this reaction are diphenyl ketone and formaldehyde.
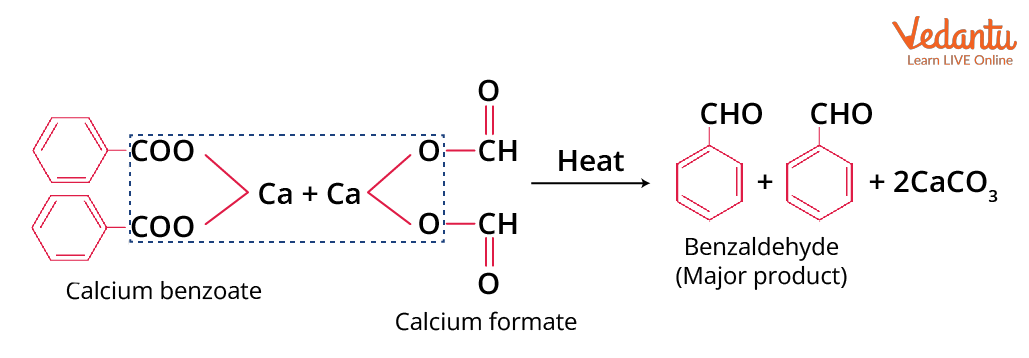
Preparation of Benzaldehyde from Calcium Benzoate and Calcium Formate
By Oxidation of Benzyl Alcohol
Treating benzyl alcohol with dilute HNO3, acidic potassium dichromate (or chromic anhydride) in acetic anhydride, or with the presence of a copper catalyst at 3500C will give:
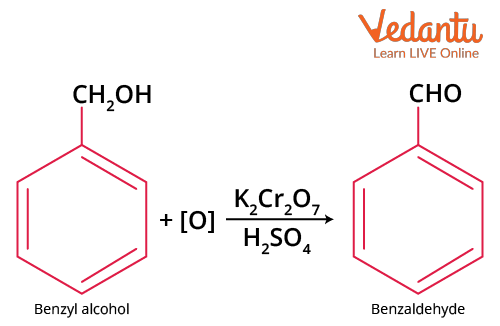
Oxidation of Benzyl Alcohol
This method is used in the commercial production of benzaldehyde.
By Oxidation of Toluene
Toluene is treated with oxygen in presence of vanadium pentoxide (V2O5) at 3500C to form benzaldehyde.
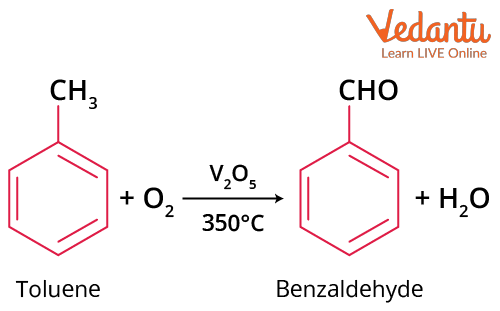
Oxidation of Toluene
For commercial purposes, oxidation of toluene can also be carried out in the presence of air diluted with nitrogen at 5000C in presence of oxides of Mn, Mo, or Zr as a catalyst. Here, nitrogen is used to prevent complete oxidation.
By Etard’s reaction
The reaction of chromyl chloride dissolved in CS2 or CCl4 with toluene in CS2 will form a brown-coloured chromium complex product, which forms benzaldehyde on decomposition with water. This method is used in laboratories for the preparation of benzaldehyde.
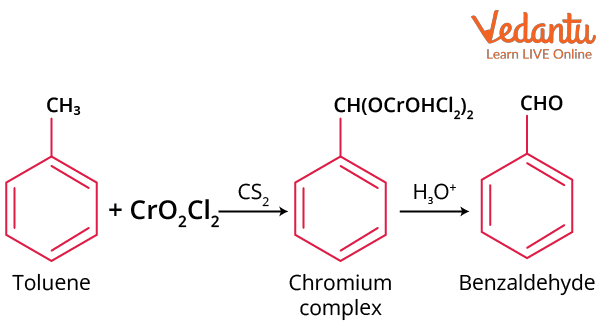
Etard’s Reaction
From toluene, benzaldehyde is also formed by oxidation of toluene with chromic oxide in acetic anhydride. This reaction will form benzylidene diacetate, which causes the production of benzaldehyde on hydrolysis with acid or alkali.
$\underset{\text{Toluene}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{3}} \underset{\left(\mathrm{CH}_{3} \mathrm{CO}\right)_{2}}{\xrightarrow{\text{CrO}_3,[\text{O}]}} \underset{\text{Benzylidene diacetate}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}\left(\mathrm{OCOCH} \mathrm{CO}_{3}\right)_{2}} \underset{{(\text { Hydrolysis })} }{\xrightarrow{\mathrm{OH}^{-} / \mathrm{H}_{2} \mathrm{O}}}\underset{\text{Benzaldehyde}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}}+2 \mathrm{CH}_{3} \mathrm{COOH}$
Gattermann-Koch Aldehyde Synthesis
Carbon monoxide and HCl gas are passed into the ether solution of benzene in presence of anhydrous aluminium chloride and cuprous chloride under high pressure of 90 atm to produce benzaldehyde.
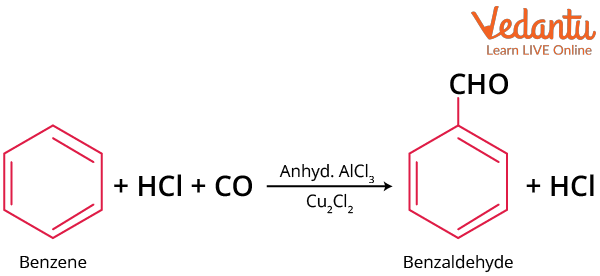
Gattermann-Koch Benzaldehyde Synthesis
Stephen’s Reduction
Partial reduction of phenyl cyanide with stannous chloride through the flow of HCl gas into the ethereal solution, followed by hydrolysis of aldimine stannic chloride with H2O, yields benzaldehyde.
$\underset{\text{phenyl cyanide}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CN}}+\mathrm{HCl} \underset{\mathrm{Ether}}{\xrightarrow{\mathrm{HCl}/ \text{SnCl}_{2}}} \underset{\text{Aldimine complex}}{\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}=\mathrm{NH}\right]_{2} \mathrm{SnCl}_{6}} \underset{\text{Boil}}{\xrightarrow{\text { H}_2\text{O}}} \underset{\text{Benzaldehyde}}{2 \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}}$
By Ozonolysis of Styrene
Styrene reacts with O3 on hydrolysis and gives benzaldehyde.
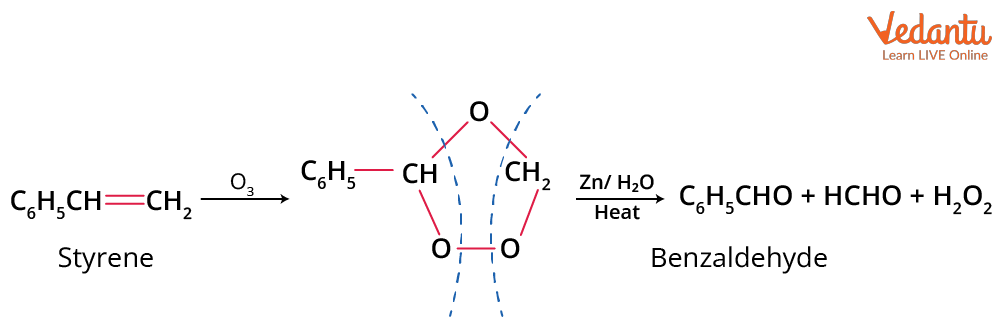
Ozonolysis of Styrene
Grignard Reaction
Treatment of phenyl magnesium bromide with reagent ethyl formate can produce benzaldehyde.
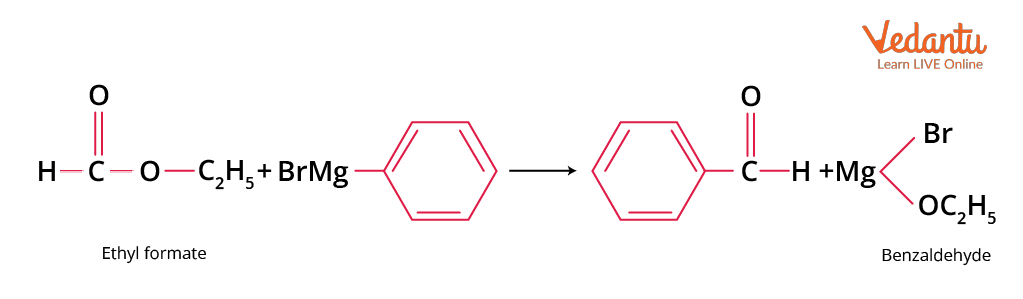
Formation of Benzaldehyde using Grignard Reagent
Physical Properties of Benzaldehyde
Colourless oily liquid with a boiling point of 1790C.
Smells like bitter almonds.
Sparingly soluble in water but highly soluble in organic solvents.
Poisonous in nature.
Some Important Chemical Reactions of Benzaldehyde
Oxidation of Benzaldehyde
Oxidation of benzaldehyde will produce benzoic acid. Thus, to prepare benzoic acid from benzaldehyde, we need to use oxidising agents like acidified K2Cr2O7, alkaline KMnO4 or dil. HNO3.
$\underset{\text{Benzaldehyde}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}} \xrightarrow{[\mathrm{O}]} \underset{\text{Benzoic acid}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}}$
Clemmensen’s Reduction
Benzaldehyde can be reduced to toluene through the use of amalgamated zinc in presence of con. HCl.
$\underset{\text { Benzaldehyde }}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}}+4 \mathrm{H} \underset{\text { conc.HCl }}{\xrightarrow{Zn-Hg}} \underset{\text{Toluene}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{3}}+\mathrm{H}_{2} \mathrm{O}$
Wolff-Kishner Reduction
Benzaldehyde can be reduced to hydrocarbon toluene in presence of excess hydrazine and a strong base such as KOH or potassium tert. butoxide, by heating at 200 degree Celsius.
$\underset{\text { Benzaldehyde }}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}}+4 \mathrm{H} \underset{200^{\circ}C}{\xrightarrow{H_2NNH_2/KOH, \text{glycol}}} \underset{\text{Toluene}}{\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{3}}+\mathrm{H}_{2} \mathrm{O}+\mathrm{N}_2$
Nitration of Benzaldehyde
Benzene ring of benzaldehyde can undergo nitration to produce m-nitrobenzaldehyde.
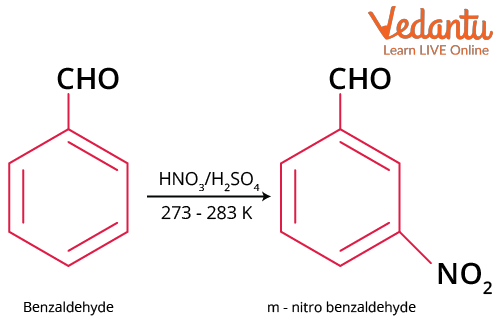
Nitration of Benzaldehyde
We know that –CHO is a deactivating group as it is an electron-withdrawing group, which pulls out the electron from a benzene ring. It decreases the electron density at ortho and para position of the benzene ring rather than at meta position. Thus, the meta position has more electron density compared to others which will lead to an attack on it by electrophile, like NO2.
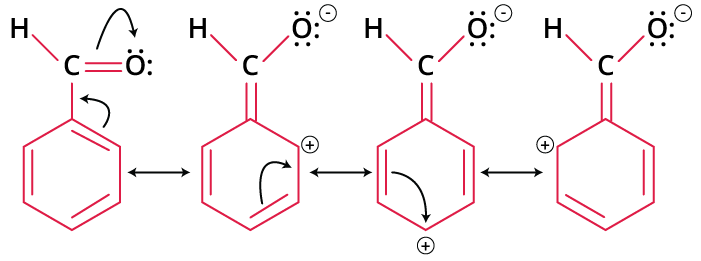
Resonance Effect of –CHO on Benzene Ring
Uses of Benzaldehyde
Used in perfumery.
Used in the manufacture of different types of dyes.
Used in the manufacture of benzoic acid, cinnamic acid, Schiff’s base, benzoyl chloride, etc.
Used as a flavouring agent for almond extract in many food items.
JEE Main 2026 Preparation Tips
Know the Syllabus: Familiarise yourself with the JEE Main syllabus and exam pattern, focusing on high-weightage topics.
Set a Schedule: Create a balanced daily study plan for all three subjects.
Practise Daily: Solve past papers and take regular mock tests to build speed and accuracy.
Revise Often: Regularly go over formulas, and concepts, and make concise notes for quick revision.
Manage Time & Accuracy: Focus on answering questions correctly within time limits.
Learn from Mistakes: Analyse each test to strengthen weak areas.
Stay Healthy: Keep a balanced routine with breaks, sleep, and hydration.
Seek Help: Use resources like Vedantu if you need extra guidance.
Stay Positive: Keep a positive mindset, and don’t stress over setbacks.
Conclusion
Benzaldehyde is an important organic compound due to its wide range of usage in food, pharmaceuticals, cosmetics etc. It can undergo numerous types of reactions, which makes it a major component of many organic reactions. Benzaldehyde is a colourless oily liquid with a boiling point of, 1790C. It has a smell like bitter almonds. It is sparingly soluble in water but highly soluble in organic solvents. Benzaldehyde is poisonous in nature. Benzaldehyde also has many industrial uses and is used as a reactant for many economic reactions.
JEE Main 2026 Subject-wise Important Chapters
The JEE Main 2026 subject-wise important chapters provide a focused strategy for Chemistry, Physics, and Maths. These chapters help students prioritise their preparation, ensuring they cover high-weightage topics for better performance in the exam.
Important Links for JEE Main and Advanced 2026
Access a curated collection of study materials and tips to excel in JEE Main and Advanced 2026, helping you prepare effectively for these prestigious engineering entrance exams.
FAQs on Chemistry Preparation of Benzaldehyde for JEE Main 2026
1. What is the IUPAC name of benzaldehyde?
According to the IUPAC system of naming for aldehyde, when a -CHO group is attached to a ring, the suffix should be carbaldehyde. In benzaldehyde, we can see that an aldehydic group is attached to a benzene ring and thus, the name of the compound should be Benzenecarbaldehyde.
However, benzaldehyde is an IUPAC accepted name and is often preferred to use. There also exist some common names for benzaldehyde which include phenyl methanol, benzenecarboxaldehyde, and benzoic aldehyde.
2. Why should benzaldehyde be fresh or distilled?
We know that the product of the oxidation of benzaldehyde is benzoic acid. It will be hard to keep any chemical free from contact with air. So, when benzaldehyde is exposed to air, it can undergo oxidation to form benzoic acid, even at room temperature.
Thus, if we do not use fresh and distilled benzaldehyde while carrying out the reactions, the produced benzoic acid can interfere with those reactions and can lead to the production of an undesired product.
3. What are the common methods for preparation of benzaldehyde?
Benzaldehyde can be prepared by the oxidation of toluene, hydrolysis of benzal chloride, and industrial methods like the Gattermann-Koch reaction.
4. How does Vedantu help in understanding the method of preparation of benzaldehyde for JEE Main 2026?
Vedantu offers detailed explanations, video lectures, and practice questions to help students grasp the various methods of preparing benzaldehyde, aligned with the JEE Main syllabus.
5. What is benzaldehyde used for in organic chemistry?
Benzaldehyde is used as a precursor for synthesising dyes, perfumes, and pharmaceuticals, and is also studied for its reactions like aldol condensation.
6. Can you describe the benzaldehyde structure?
Benzaldehyde has a benzene ring attached to a formyl group (-CHO), making it an aromatic aldehyde with the molecular formula C7H6O.
7. What is the molecular weight of benzaldehyde?
Benzaldehyde molar mass is 106.12 g/mol, calculated based on its molecular formula, C7H6O.benzyl chloride to benzaldehyde its well explained by our Vedantu experts, Students can visit and download the other important study materials available on the Vedantu website.
8. How does benzaldehyde structure and uses help in JEE Main 2026 preparation?
Understanding the structure of benzaldehyde aids in learning its chemical properties and uses, which are often part of organic reaction questions in JEE Main Exam.
9. How is benzyl chloride converted to benzaldehyde?
Benzyl chloride is converted to benzaldehyde by hydrolysis in the presence of aqueous NaOH, a commonly studied reaction in organic chemistry.
10. What is the benzaldehyde molar mass, and why is it important?
The benzaldehyde molar mass is 106.12 g/mol. It’s crucial for stoichiometric calculations in reactions and for solving numerical problems in JEE Main.
11. Why is the preparation of benzaldehyde significant for JEE Main organic chemistry?
The preparation of benzaldehyde demonstrates key organic reactions like oxidation and substitution, making it an important topic for understanding reaction mechanisms in JEE Main.

































