




Best Reagents for Converting Alcohol to Haloalkane (With Mechanisms)
The Preparation of Haloalkane from Alcohol is a foundational organic reaction in JEE Main Chemistry, crucial for understanding nucleophilic substitution and practical laboratory conversions. This transformation involves replacing the –OH group in alcohols with a halogen atom (Cl, Br, or I) to synthesize haloalkanes or alkyl halides. Mastery of this topic boosts conceptual clarity in organic pathways, mechanisms, and reagent selection—skills directly tested in JEE.
Key Concepts: What are Haloalkanes and Why Prepare Them from Alcohols?
Haloalkanes are saturated organic compounds where one or more hydrogen atoms of an alkane are substituted with a halogen. Their general formula is R–X, where R is an alkyl group and X is a halogen. Converting alcohols to haloalkanes is the easiest and most controlled laboratory method, offering predictable yields and cleaner products, making it a preferred JEE conversion reaction.
Main Methods for Preparation of Haloalkane from Alcohol
Multiple reagents convert alcohols into haloalkanes, each suited for different practical needs and types of alcohols. Main methods you must know for JEE are:
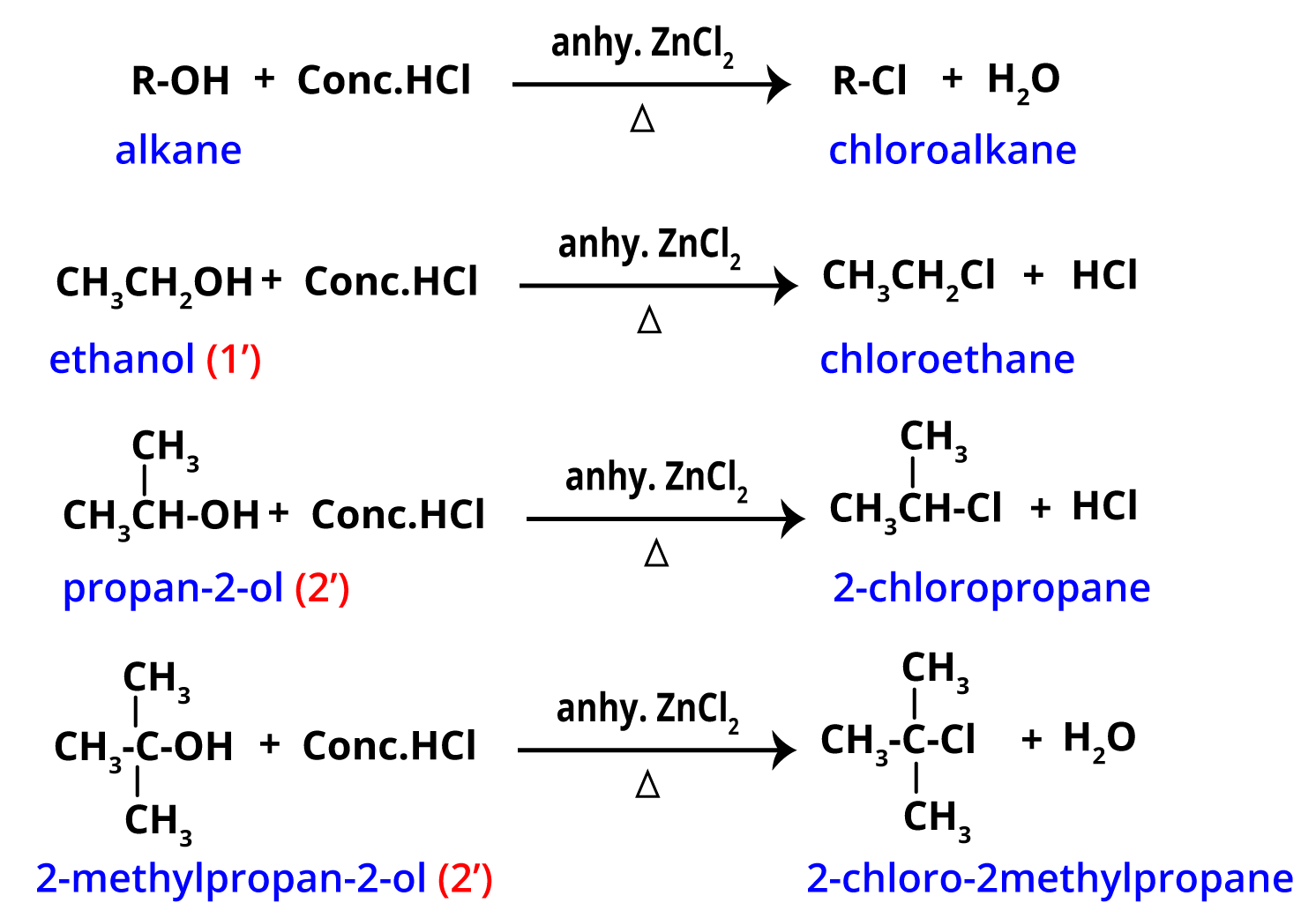
- Halogen Acids Method (HX): Alcohol reacts with concentrated hydrochloric (HCl), hydrobromic (HBr), or hydroiodic (HI) acid. For alcohol to chloroalkane conversions, Lucas reagent (conc. HCl + anhydrous ZnCl2) is used, especially for primary and secondary alcohols.
- Phosphorus Halides (PX3, PX5): Alcohol is treated with phosphorus pentachloride (PCl5), trichloride (PCl3), or their bromo/iodo analogs to yield the corresponding haloalkane. PBr3 and PI3 are commonly generated in situ using red phosphorus and halogen.
- Thionyl Chloride (SOCl2): Specially favored for laboratory synthesis. Alcohol reacts with SOCl2 producing chloroalkane, SO2, and HCl. Both SO2 and HCl are gases, which escape, leaving pure haloalkane.
The method chosen depends on the alcohol’s structure (primary, secondary, tertiary) and desired halogen (Cl, Br, I).
General Reaction Equations for Preparation of Haloalkanes from Alcohols
Here are stepwise reactions showcasing common laboratory conversions for JEE:
- Alcohol + HCl (with ZnCl2): R–OH + HCl → R–Cl + H2O (ZnCl2 as catalyst; Lucas test for differentiation of alcohols)
- Alcohol + SOCl2: R–OH + SOCl2 → R–Cl + SO2 + HCl (no purification needed)
- Alcohol + PBr3: 3R–OH + PBr3 → 3R–Br + H3PO3 (for bromoalkanes)
- Alcohol + PI3: 3R–OH + PI3 → 3R–I + H3PO3
- Alcohol + PCl5: R–OH + PCl5 → R–Cl + POCl3 + HCl
The best method for preparing haloalkane from alcohol is usually with SOCl2 because the byproducts are volatile and do not contaminate the product.
Mechanisms Involved: SN1 vs SN2 in Alcohol to Haloalkane Conversion
The alcohol to haloalkane mechanism depends on the nature of the alcohol:
- Primary alcohols: Undergo SN2 mechanism—concerted, no stable carbocation intermediate. Transition state involves simultaneous bond making and breaking.
- Tertiary alcohols: Follow SN1 mechanism—two steps (carbocation formed, then halide attacks). Fast for tertiary due to greater carbocation stability.
- Secondary alcohols: Can follow SN1 or SN2 pathway based on conditions.
Steric hindrance and solvent choice can tip the balance between SN1 and SN2 for specific conversions in JEE questions. Always check which carbocation (if any) can form.
Comparison: Choosing the Right Reagent to Prepare Haloalkanes
In competitive exams, you are often asked: Which is the most efficient and clean reagent for converting alcohol to haloalkane? Here’s a quick comparison:
| Reagent | Features | Best Use |
|---|---|---|
| SOCl2 | Byproducts (SO2, HCl) are gaseous; pure product | Preferred for lab prep of alkyl chlorides |
| PCl5, PCl3 | Multiple byproducts; needs purification | Bulk synthesis, all types of alcohol |
| HX + ZnCl2 | Lucas test; slow for primary alcohols | Distinguishing alcohol types, quick for tertiary |
Always choose SOCl2 method for cleaner laboratory-scale preparation of haloalkane from alcohol in JEE-style conversion problems.
Special Case: Preparation of Alkyl Iodides (RI) from Alcohol
Alkyl iodides are not formed efficiently with HI as the acid is unstable. The best method is halogen exchange (Finkelstein reaction):
- Convert alcohol to alkyl bromide (R–Br) using HBr or PBr3.
- Treat R–Br with NaI in acetone: R–Br + NaI → R–I + NaBr (ppt).
This two-step process ensures high yields, making it an essential note for the preparation of RI from alcohol in JEE conversions.
Practice and Application: JEE-Style Questions on Alcohol to Haloalkane Conversion
Be ready for conversion, mechanism, and reagent choice questions. Typical formats in JEE include:
- Identify the best reagent for converting ethanol to bromoethane.
- Given a SN2 pathway, which alcohol yields the corresponding haloalkane fastest?
- Predict the product and mechanism when 2-propanol reacts with SOCl2.
- Arrange in order of reactivity with Lucas reagent: ethanol, isopropanol, tert-butanol.
- Write stepwise equations for the conversion of butan-1-ol to 1-iodobutane.
For more practice, solve organic compounds containing halogens mock test and access other JEE main chemistry Q&A sets for targeted preparation.
Quick-Revision Cheat Sheet: Alcohol to Haloalkane Conversion Table
Revise all main points in seconds before the exam using this summary chart:
| Alcohol | Reagent | Conditions | Haloalkane | Byproducts |
|---|---|---|---|---|
| R–OH | SOCl2 | Dry, RT | R–Cl | SO2, HCl (gases) |
| R–OH | PBr3 | Red P, Br2 | R–Br | H3PO3 |
| R–OH | HCl + ZnCl2 | Heat (primary/secondary) | R–Cl | H2O |
| R–Br | NaI (acetone) | Room temperature | R–I | NaBr (ppt) |
Link your preparation with related topics such as organic compounds containing halogens and nucleophilic substitution reactions for JEE convergence. For more on alcohol reactivity and practice, check out difference between alcohol and phenol and methods of preparation of haloalkanes and haloarenes.
With these conversion reactions, equations, and mechanism tricks, you’ll be ready for any JEE Main question on Preparation of Haloalkane from Alcohol. For topic-wise tests and doubt-solving, use Vedantu’s free resources and stay exam-ready!
FAQs on Preparation of Haloalkane from Alcohol: Complete Stepwise Guide
1. How are haloalkanes prepared from alcohols?
Haloalkanes are prepared from alcohols by replacing the -OH group of alcohols with a halogen atom using specific reagents and conditions. The main methods include:
- Treating alcohols with SOCl2 (thionyl chloride) to make alkyl chlorides efficiently
- Using PCl5 or PCl3 for chlorination
- Reacting with HBr or HI for alkyl bromides and iodides
- Through the Lucas reagent (HCl + ZnCl2), especially for distinguishing alcohol types
2. Which is the best reagent for converting alcohol to haloalkane?
The best reagent for converting alcohol to haloalkane is thionyl chloride (SOCl2), especially for producing alkyl chlorides. This method is preferred because:
- It gives a high yield of haloalkane
- The side products (SO2 and HCl) are gases and escape, leaving a pure product
- No extra purification is needed
3. What is the role of SOCl2 in the preparation of haloalkanes?
SOCl2 (thionyl chloride) is an efficient chlorinating agent for preparing alkyl chlorides from alcohols. Its importance includes:
- It replaces the alcohol’s –OH group with a –Cl atom
- Produces only gaseous by-products (SO2, HCl), making product isolation easy
- Minimizes side reactions and provides a clean conversion
4. How do you prepare alkyl iodide (RI) from alcohol?
To prepare alkyl iodide (RI) from an alcohol, the usual method is:
- Treating alcohol with red phosphorus (P) and iodine (I2)
- This forms PI3 in situ, which reacts with the alcohol to give RI
- Alternatively, reacting alcohol with NaI + concentrated H2SO4 will generate the corresponding alkyl iodide
5. What is the mechanism of alcohol to haloalkane conversion?
The mechanism of alcohol to haloalkane conversion typically involves a nucleophilic substitution reaction (either SN1 or SN2 pathway):
- SN1 Mechanism: For tertiary (and some secondary) alcohols, forming a carbocation intermediate
- SN2 Mechanism: For primary alcohols, where halide ion directly displaces the –OH group
- Choice of mechanism depends on the structure of the alcohol and the reaction conditions
6. Why is SOCl2 preferred over PX5 for making haloalkanes?
SOCl2 is preferred over PCl5 because:
- It produces only gaseous side products (SO2 and HCl), resulting in easy purification
- Reaction is clean with almost quantitative yield
- No extra purification steps are needed as opposed to PCl5, which forms solid by-products
7. Can tertiary alcohols be converted to haloalkanes by the same methods as primary alcohols?
Yes, tertiary alcohols can be converted to haloalkanes, but the mechanism differs:
- Tertiary alcohols typically undergo an SN1 mechanism, forming a stable carbocation intermediate
- Primary alcohols usually react via SN2 mechanism
- All common methods (SOCl2, PX5, HX) work, but reactivity and side reactions may vary
8. What are the different methods for preparing haloalkanes from alcohols?
There are several important methods for preparing haloalkanes from alcohols:
- Using SOCl2 for chlorides
- Reacting with PCl3 or PCl5 for chlorides
- Treating with HCl + ZnCl2 (Lucas reagent) for chlorides
- Using PBr3 or HBr for bromides
- Using PI3 (from P + I2) or HI for iodides
9. How do you remember the reagents and conditions for alcohol to haloalkane conversion?
To remember reagents and conditions for alcohol to haloalkane conversion, use:
- Mnemonics (e.g., ‘SOCl2 is for chlorides, PBr3 for bromides’)
- Quick-reference tables grouping alcohols and products
- Practice with exam-style MCQs and flowcharts
10. Why can't ordinary halogen (Cl2) convert alcohol directly into haloalkane?
Ordinary halogen (Cl2) cannot convert alcohol directly into a haloalkane because:
- The -OH group in alcohol is a poor leaving group and unreactive to simple halogen substitution
- Specific reagents (SOCl2, PX5, HX) are required to activate the alcohol for substitution
- Direct reaction with Cl2 would result in no conversion under normal conditions























