Reactions of Benzene Revision Notes for JEE Advanced 2026: Free PDF Download
FAQs on JEE Advanced 2026 Revision Notes for Reactions of Benzene
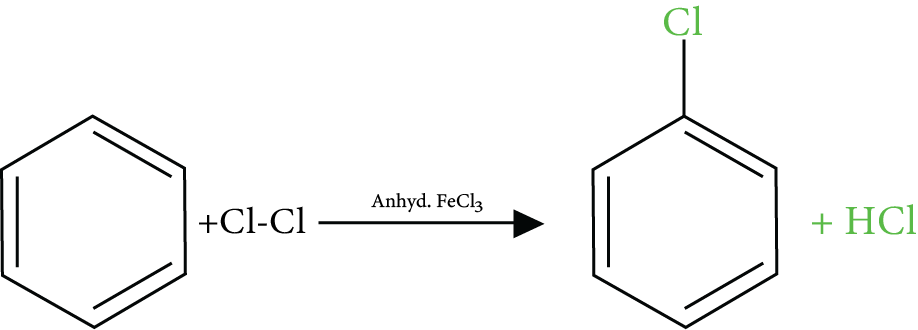
1. What is halogenation?
The organic reaction where a halogen is introduced into the molecule of an organic compound through a chemical reaction is called halogenation. It can be achieved using different reagents and chemical conditions.
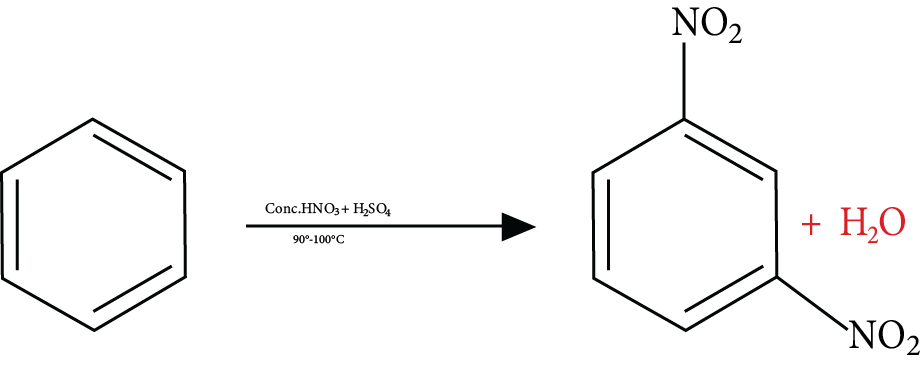
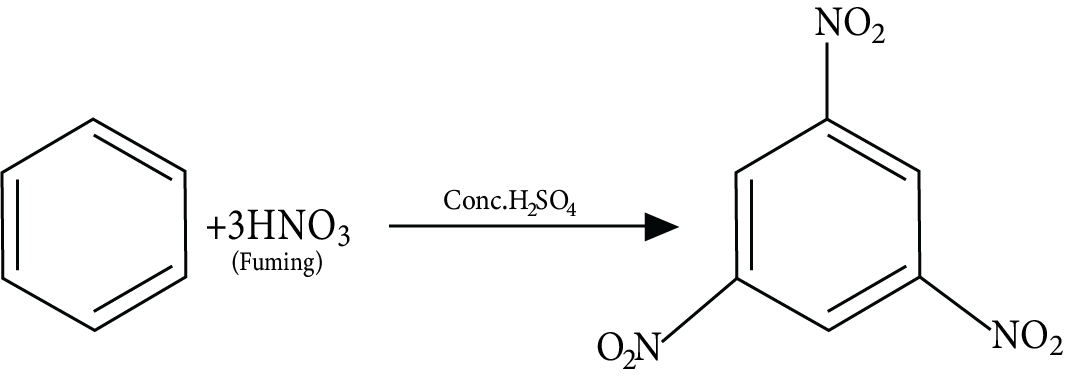
2. Which functional group is added in a nitration reaction?
In a nitration reaction, nitrate (NO2) is added to an organic compound. This functional group attaches to one of the carbon atoms of an organic compound.
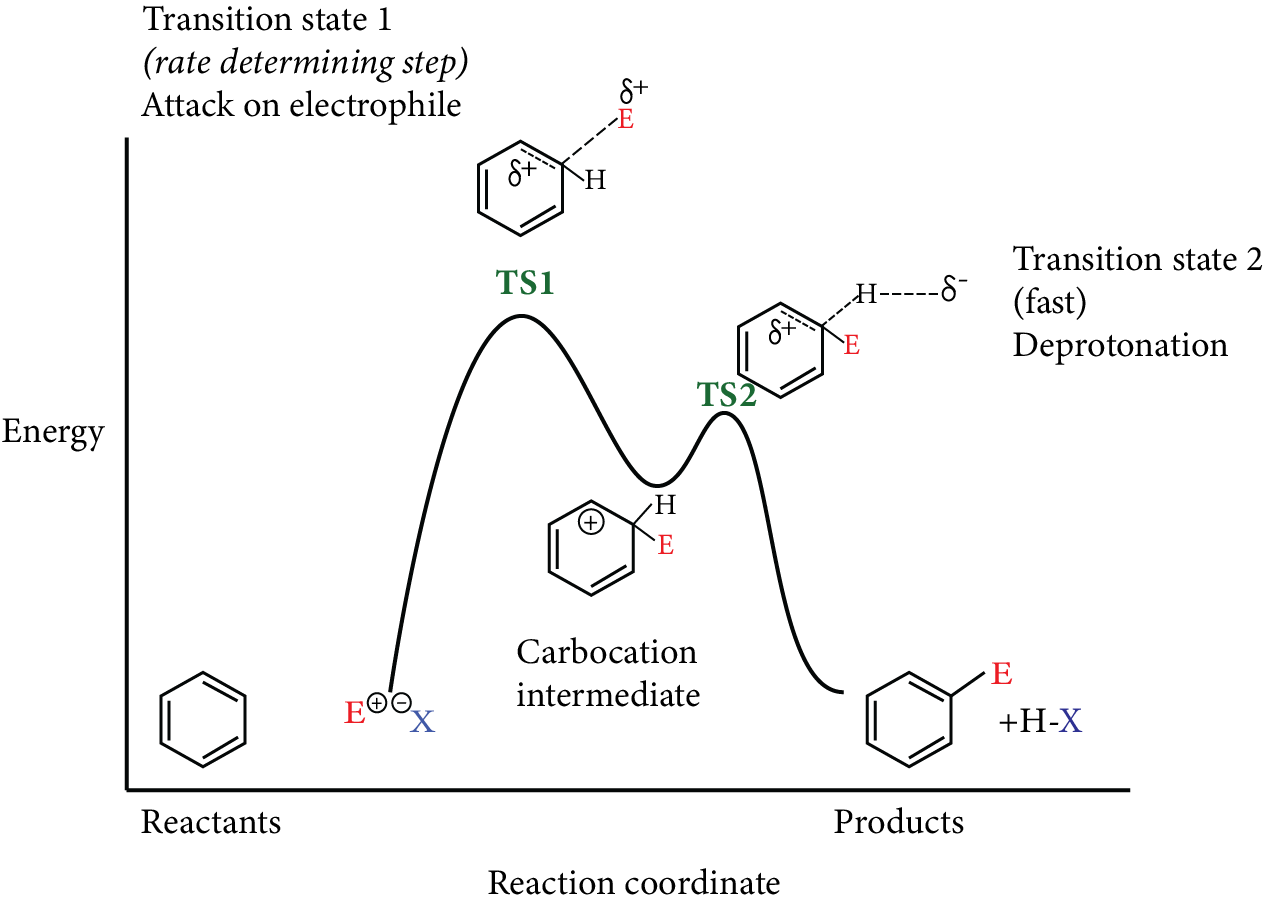
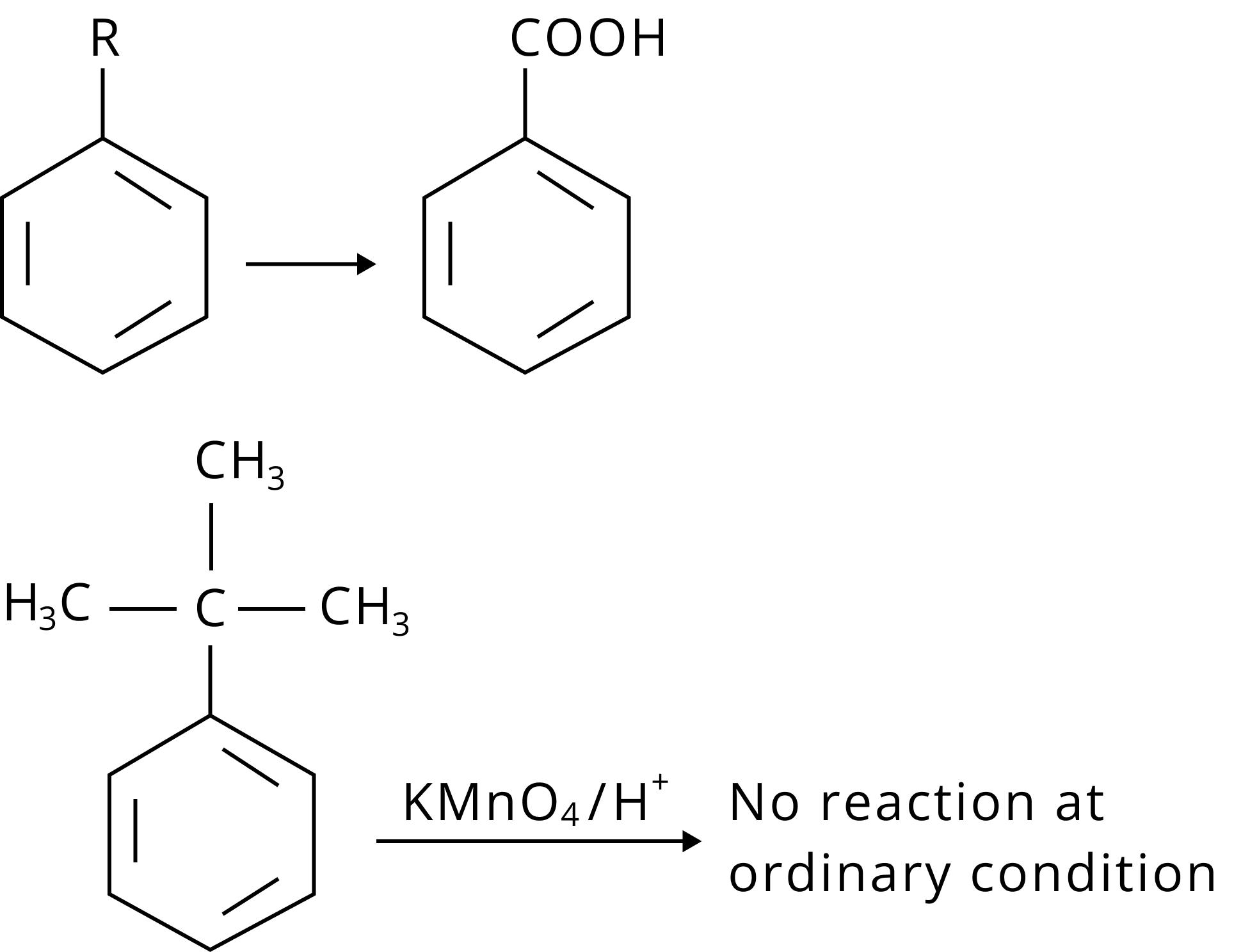
3. What is benzene?
Benzene is a 6-carbon aromatic or cyclic organic compound. In this compound, the 6 carbon atoms form a hexagonal ring with alternate double bonds among the two carbon atoms. It also has a hydrogen atom attached to each of the carbon atoms.
4. What kind of electrons are there in a benzene ring?
The electrons in a benzene ring are sp2 hybridised in nature. These π electrons remain delocalized or spread among all the carbon atoms constituting the ring.