
An example of soap is:
A. \[{{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{35}}}}{\text{COONa}}\]
B. ${\text{C}}{{\text{H}}_{\text{3}}}{\text{COONa}}$
C. ${\text{C}}{{\text{H}}_{\text{3}}}{\text{ONa}}$
D. \[{{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{35}}}}{\text{COO}}{{\text{C}}_2}{{\text{H}}_5}\]
Answer
233.1k+ views
Hint: Soaps are a class of surfactants which are chemical substances that concentrate at the surface of the solution, form surface films, reduce surface tension of the solution and emulsify grease.
Soaps are actually potassium or sodium salts of higher fatty acids such as lauric acid $\left( {{{\text{C}}_{{\text{11}}}}{{\text{H}}_{{\text{23}}}}{\text{COOH}}} \right)$ or palmitic acid $\left( {{{\text{C}}_{{\text{15}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}} \right)$ .
Complete step by step answer:
Soaps are surface active agents and they can remove dirt and dust by emulsifying grease. So, they act as cleansing agents. The molecule of soaps consists of two characteristic groups, one of which is water soluble or hydrophilic and the other is oil soluble or lipophilic.
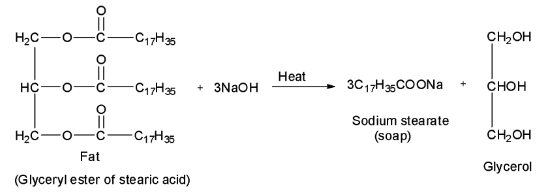
Soaps are formed by heating fat or oil, or in other words, glyceryl esters of fatty acids with aqueous sodium hydroxide solution. Alcohol is also formed in this process. This process of conversion of fats, oils or lipids into soap and alcohol is known as saponification reaction.
Now, out of the given four options, only the first option is a sodium salt of a higher fatty acid, i.e. \[{{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{35}}}}{\text{COONa}}\] is a sodium salt of a higher fatty acid called stearic acid having the formula \[{{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{35}}}}{\text{COOH}}\] . Therefore, it is a soap called sodium stearate. It is formed by the saponification of glyceryl ester of stearic acid. Thus, option A is correct.
${\text{C}}{{\text{H}}_{\text{3}}}{\text{COONa}}$ is sodium acetate, ${\text{C}}{{\text{H}}_{\text{3}}}{\text{ONa}}$ is sodium methoxide and \[{{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{35}}}}{\text{COO}}{{\text{C}}_2}{{\text{H}}_5}\] is an ester of a higher fatty acid. Therefore, they are not soaps and so the options B, C and D are not correct.
Note:
Soap is a good cleansing agent and is completely biodegradable. Thus, it does not create any pollution problems.
But there are two disadvantages. One of them is soaps cannot be used in hard water because calcium and magnesium ions present in hard water produces curdy white precipitates of calcium and magnesium salts of fatty acids. These get precipitated as scum and some parts of soap is wasted.
Another disadvantage is soaps cannot be used in acidic solutions because acids precipitate the insoluble free fatty acids which adhere to the fabric and thereby lowers the ability to remove oil and grease from the fabric.
Soaps are actually potassium or sodium salts of higher fatty acids such as lauric acid $\left( {{{\text{C}}_{{\text{11}}}}{{\text{H}}_{{\text{23}}}}{\text{COOH}}} \right)$ or palmitic acid $\left( {{{\text{C}}_{{\text{15}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}} \right)$ .
Complete step by step answer:
Soaps are surface active agents and they can remove dirt and dust by emulsifying grease. So, they act as cleansing agents. The molecule of soaps consists of two characteristic groups, one of which is water soluble or hydrophilic and the other is oil soluble or lipophilic.
Soaps are formed by heating fat or oil, or in other words, glyceryl esters of fatty acids with aqueous sodium hydroxide solution. Alcohol is also formed in this process. This process of conversion of fats, oils or lipids into soap and alcohol is known as saponification reaction.
Now, out of the given four options, only the first option is a sodium salt of a higher fatty acid, i.e. \[{{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{35}}}}{\text{COONa}}\] is a sodium salt of a higher fatty acid called stearic acid having the formula \[{{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{35}}}}{\text{COOH}}\] . Therefore, it is a soap called sodium stearate. It is formed by the saponification of glyceryl ester of stearic acid. Thus, option A is correct.

${\text{C}}{{\text{H}}_{\text{3}}}{\text{COONa}}$ is sodium acetate, ${\text{C}}{{\text{H}}_{\text{3}}}{\text{ONa}}$ is sodium methoxide and \[{{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{35}}}}{\text{COO}}{{\text{C}}_2}{{\text{H}}_5}\] is an ester of a higher fatty acid. Therefore, they are not soaps and so the options B, C and D are not correct.
Note:
Soap is a good cleansing agent and is completely biodegradable. Thus, it does not create any pollution problems.
But there are two disadvantages. One of them is soaps cannot be used in hard water because calcium and magnesium ions present in hard water produces curdy white precipitates of calcium and magnesium salts of fatty acids. These get precipitated as scum and some parts of soap is wasted.
Another disadvantage is soaps cannot be used in acidic solutions because acids precipitate the insoluble free fatty acids which adhere to the fabric and thereby lowers the ability to remove oil and grease from the fabric.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




