
Azide ion (${{N}_{3}}^{-}$) exhibits an N-N bond order of 2 and may be represented by resonance structures I, II and III given below. Select correct statements.
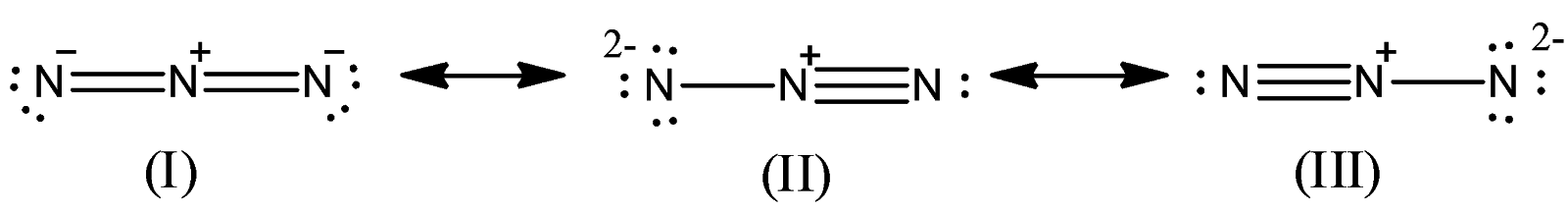
(A) Structures I and II make greater contributions than III
(B) Structures II and III make greater contributions than I
(C) Structures I and III make greater contributions than II
(D) All the above three structures make equal contributions.
Answer
233.1k+ views
Hint: Here, contributions they are talking about is towards the reactivity of the azide ion. The resonance structure that can easily remove nitrogen gas from its structure will be comparatively less stable.
Complete step by step solution:
Here, we are being asked which of the given resonance structures of azide ion give major contributions. Here, they are talking about contributions towards reactivity of the azide ion.
-We know that stable compounds have less energy compared to unstable ones and so they have comparatively less reactivity. Unstable compounds have high reactivity and they react with other compounds easily than the stable compounds.
-So, we can say that the more unstable resonance structures of azide will react more often and will give more contributions towards reactivity of azides.
-The resonance structures II and III have an N-N triple bond while structure I have two N-N double bonds.
-The structure II and III are more unstable because it contains a nitrogen atom with -2 charge on it which is also called nitrene. So, both II and III will give $N\equiv N$ (Nitrogen gas) upon reaction and so they are more reactive than structure I. Structure I does not have nitrogen with -2 charge and two nitrogen there has a charge of -1.
-So, we can say that structure II and III are less stable and more reactive. So, they will give more contributions than structure I.
So, the correct answer is (B).
Note: Do not assume that as two nitrogen atoms are charged in structure I so it will be more unstable. Nitrogens with -2 charge are more unstable than the nitrogen atoms with -1 charge on it.
Complete step by step solution:
Here, we are being asked which of the given resonance structures of azide ion give major contributions. Here, they are talking about contributions towards reactivity of the azide ion.
-We know that stable compounds have less energy compared to unstable ones and so they have comparatively less reactivity. Unstable compounds have high reactivity and they react with other compounds easily than the stable compounds.
-So, we can say that the more unstable resonance structures of azide will react more often and will give more contributions towards reactivity of azides.
-The resonance structures II and III have an N-N triple bond while structure I have two N-N double bonds.
-The structure II and III are more unstable because it contains a nitrogen atom with -2 charge on it which is also called nitrene. So, both II and III will give $N\equiv N$ (Nitrogen gas) upon reaction and so they are more reactive than structure I. Structure I does not have nitrogen with -2 charge and two nitrogen there has a charge of -1.
-So, we can say that structure II and III are less stable and more reactive. So, they will give more contributions than structure I.
So, the correct answer is (B).
Note: Do not assume that as two nitrogen atoms are charged in structure I so it will be more unstable. Nitrogens with -2 charge are more unstable than the nitrogen atoms with -1 charge on it.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




