
Benzyne intermediate is not observed in:
(A) 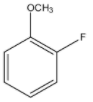
(B) 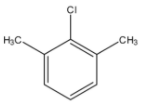
(C) 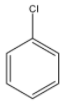
(D) 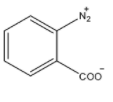
Answer
233.1k+ views
Hint: The structure requires a leaving group and a hydrogen atom at the adjacent carbon to the carbon which is bearing the leaving group. A good leaving is usually a weak base. Benzyne involves a C-C triple bond in its structure.
Complete step by step solution:
-Benzyne is a six-member hydrocarbon ring involving two double bonds and a C-C triple bond.
-In the mechanism of formation of benzyne, it is necessary for the structure to have a good leaving group. Alongside a good leaving group, it is also necessary to have a hydrogen atom at adjacent carbon which can leave to form benzyne. In short, we can show the formation of benzyne as
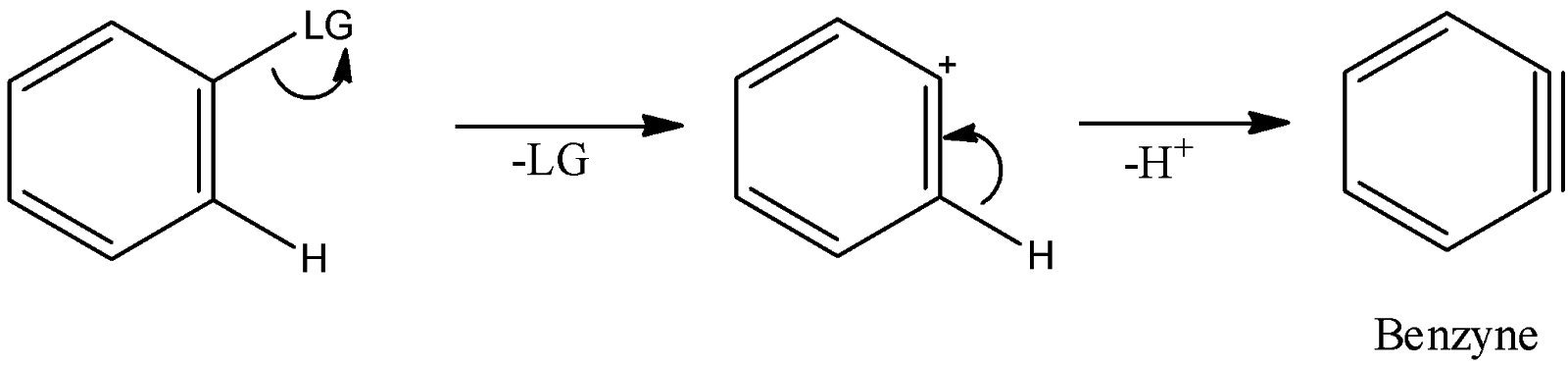
Here LG is leaving the group.
Let’s see the compounds given in all the options in order to find which one will not give benzyne.
(A)
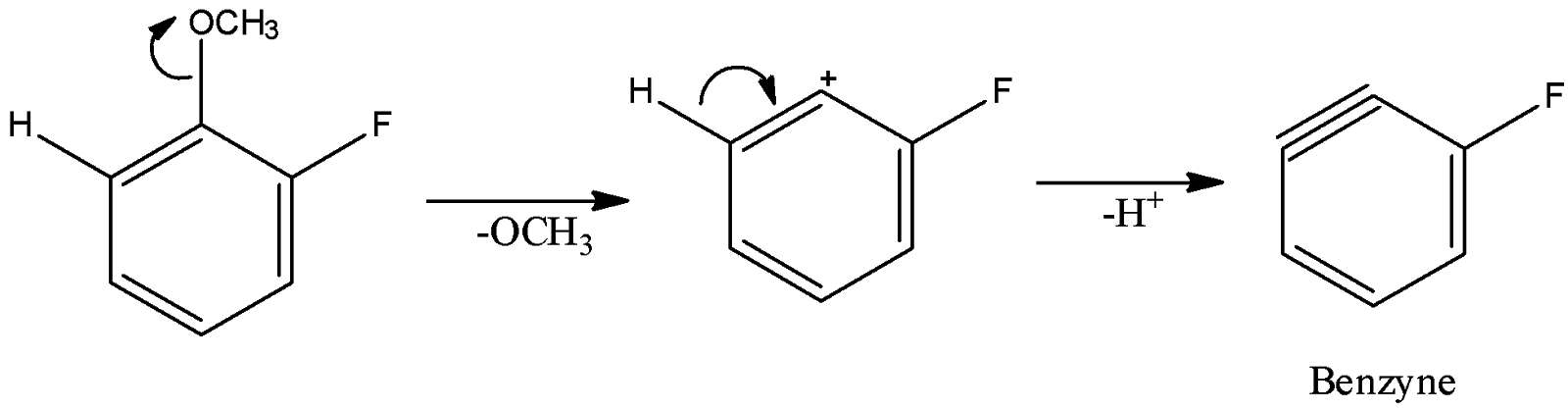
So, in this case, benzyne intermediate can be formed as $ - OC{H_3}$ is a good leaving group and there is a hydrogen atom at the adjacent carbon of the leaving group.
(B)
In this structure, there is a chlorine group which can act as a leaving group but there is no hydrogen atom with the adjacent carbon bearing the leaving group. So, this compound cannot give benzyne intermediate.
(C)
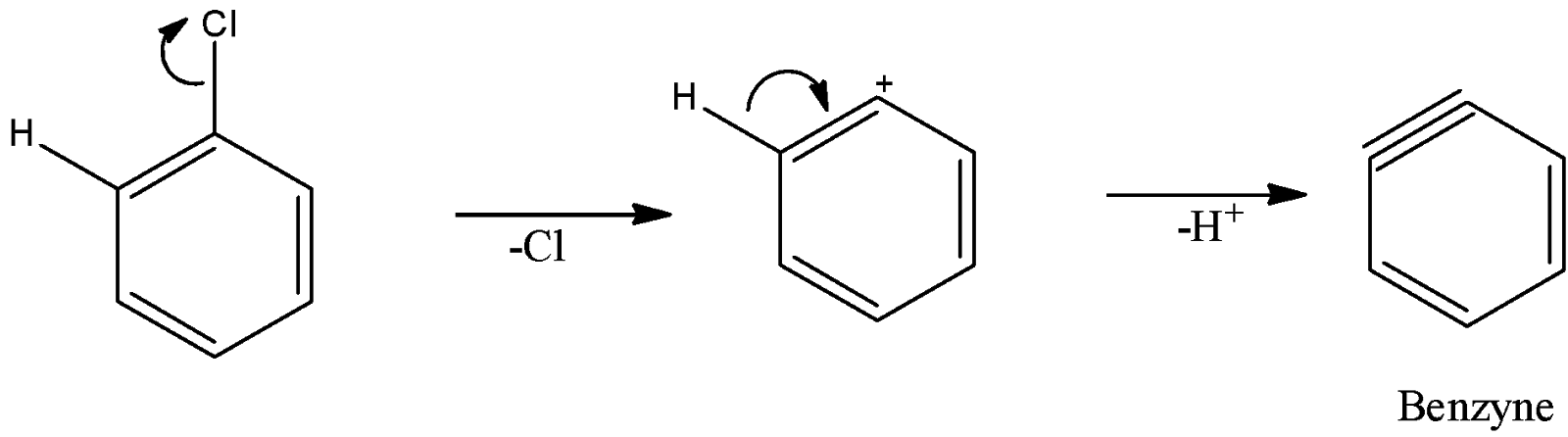
Here, we can see that there is the presence of adjacent hydrogen alongside the leaving group, chlorine. So, benzyne can be formed.
(D)
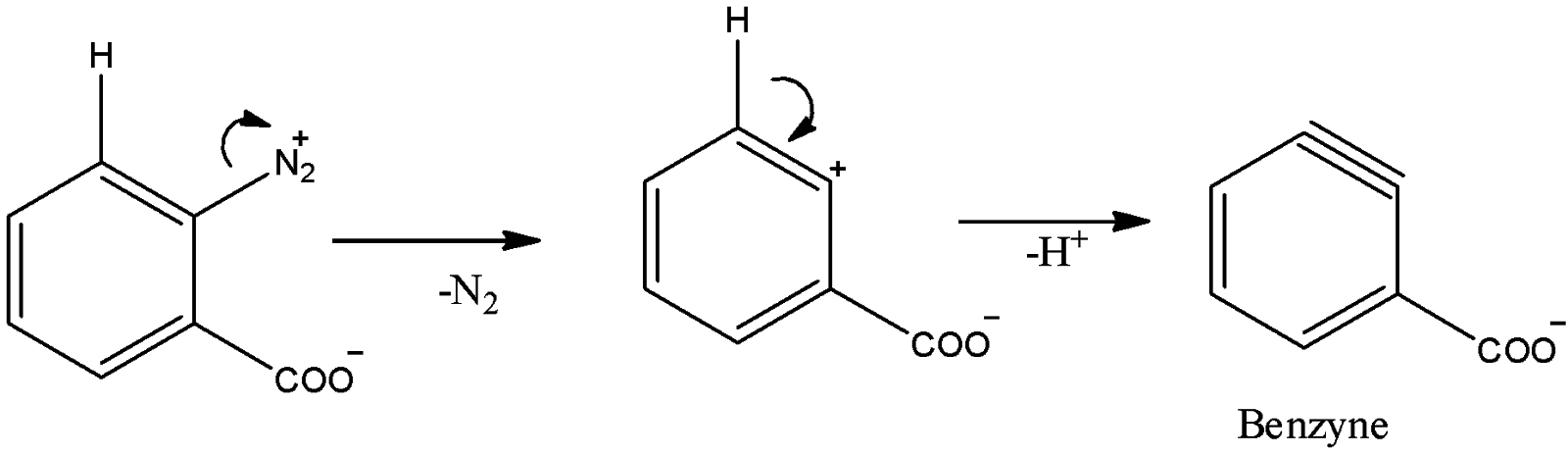
Here, ${N_2}^ + $ group can act as a good leaving group and there is a hydrogen atom at adjacent carbon bearing the leaving group. So, benzyne formation is also possible here.
Therefore, the correct answer is (B).
Note: Remember that in option (A), there are a total of two groups –F and methoxy group. Here, -F is a strong base and methoxy group is a weaker base. So, as weaker bases are good leaving groups, it will leave and form benzyne in suitable conditions.
Complete step by step solution:
-Benzyne is a six-member hydrocarbon ring involving two double bonds and a C-C triple bond.
-In the mechanism of formation of benzyne, it is necessary for the structure to have a good leaving group. Alongside a good leaving group, it is also necessary to have a hydrogen atom at adjacent carbon which can leave to form benzyne. In short, we can show the formation of benzyne as

Here LG is leaving the group.
Let’s see the compounds given in all the options in order to find which one will not give benzyne.
(A)

So, in this case, benzyne intermediate can be formed as $ - OC{H_3}$ is a good leaving group and there is a hydrogen atom at the adjacent carbon of the leaving group.
(B)
In this structure, there is a chlorine group which can act as a leaving group but there is no hydrogen atom with the adjacent carbon bearing the leaving group. So, this compound cannot give benzyne intermediate.
(C)

Here, we can see that there is the presence of adjacent hydrogen alongside the leaving group, chlorine. So, benzyne can be formed.
(D)

Here, ${N_2}^ + $ group can act as a good leaving group and there is a hydrogen atom at adjacent carbon bearing the leaving group. So, benzyne formation is also possible here.
Therefore, the correct answer is (B).
Note: Remember that in option (A), there are a total of two groups –F and methoxy group. Here, -F is a strong base and methoxy group is a weaker base. So, as weaker bases are good leaving groups, it will leave and form benzyne in suitable conditions.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




