




Introduction to Carbenes
Carbenes are transient carbon intermediates which are neutral. They have two unshared valence electrons along with a neutral carbon atom having a valency of two. They are represented as R-(C:)-R’ or R=C or R2C: Here, R represents the substituent group or hydrogen atoms. Based on their structure, they can either be singlet or triplet carbenes.
Methylene (CH2) is the simplest carbene.
Structure of Carbenes
Carbenes are short-lived reaction intermediates. Owing to electron deficiency, they are highly reactive but neutral. The nature of the substituent group attached to the carbon atom also affects the chemical reactivity and the electron structure of the compound. Due to the presence of only six electrons in their outer shell, they show electrophilicity, the strength of which increases with the presence of an electron-withdrawing group attached to the carbene. However, in cases where very strong donor groups are attached to the carbene, it behaves as a nucleophile.
Speaking of the carbene structure, it has six electrons in its outer orbital and contains a nonbonding electron pair with a formal charge of zero. On the basis of their structure, carbenes can be classified as singlet and triplet carbenes. They can either be linear or angular (bent).
Singlet and Triplet Carbenes
Carbenes have two bonding electrons as well as two non-bonding electrons. Depending on whether the non-bonding electrons of a carbene are present in the same orbital or different orbitals, they are divided into two classes - singlet and triplet carbene.
Carbenes have sp2 hybridised carbon atoms based on the valence bond theory. The two nonbonding electrons of the carbene must be placed in the vacant orbitals. Let us now define singlet carbene. In this, both the nonbonding electrons are placed in the same orbital and have opposite spins. On the basis of Hund’s law, when the electrons are placed in different orbitals but have parallel spins, they are called the triplet carbene. The carbene structure in triplet carbene can either be bent or linear.
Since the single carbene has an electron pair, it is paramagnetic. On the other hand, since the triplet carbene contains unpaired electrons, it is diamagnetic. Examples of singlet carbenes: Methoxymethylene (CH3O-C-H), Chloromethylene (Cl-C-H), and Phenylchloromethylene (C6H5-C-Cl). Examples of triplet carbenes: Methylene (H-C-H), Phenylmethylene (C6H5-C-H), and Diphenylmethylene (C6H5-C-C6H5).
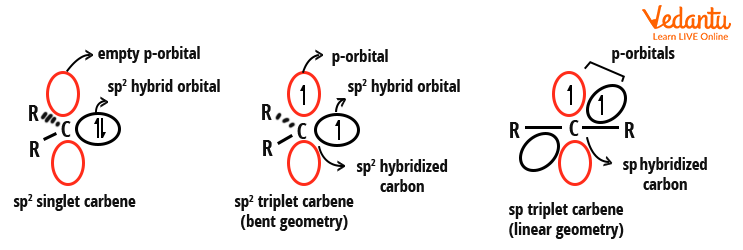
Structures of singlet and triplet carbene
Differences Between Singlet and Triplet Carbenes
Production of Carbenes
Elimination and fragmentation reactions are commonly used for the synthesis of carbenes. When groups linked to carbon atoms are broken as a result of photolysis, thermolysis, or reactivity with metals, carbenes are generated as intermediate products.
1. Carbenes from Diazo Compounds
Diazo compounds (RR’C=N2) are one of the major precursors of carbene. Diazo compounds having 1,3-dipolar structures like diazomethane can be converted into their related carbenes by the removal of nitrogen gas upon heating. Various metal complexes can act as catalysts for this process. These include dirhodium, copper, and iron.
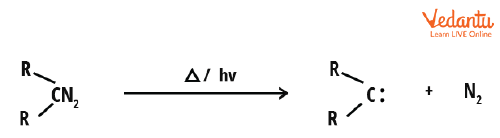
Generation of carbene from diazo compounds
2. Carbenes by 𝝰-Elimination
𝝰-elimination are those elimination reactions in which both the proton and the leaving group are present on the same carbon atom. This process involves the use of a strong base such as NaOH that removes an acidic proton present adjacent to the electron-withdrawing group, resulting in the formation of carbanion. When the leaving group is lost from the carbanion, a carbene is formed.
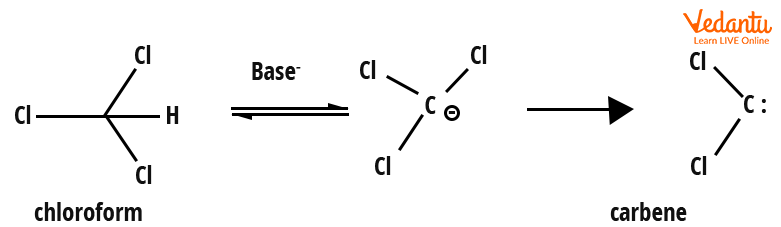
Generation of carbene by 𝝰-elimination
Application of Carbenes
The major application of carbenes is that they act as really efficient organocatalysts and transition metal catalysts for the production of complex compounds. Hence, they play a major role in the field of organometallic Chemistry. It is extensively used in the industrial production of the precursor of Teflon called tetrafluoroethylene (TFE). Difluorocarbene is the intermediate formed in this reaction.
Important Questions
1. Why is carbene a neutral species?
Ans. According to the electronic theory of bonding, bonds between atoms are formed by the sharing of electrons. A carbene, according to this theory, is a compound in which only two of the four valence, or bonding, electrons of a carbon atom are actively bonding with other atoms. In contrast, in multiple bonded compounds such as hydrogen cyanide, all four valence electrons of the atoms are involved in bonds with other atoms. Carbenes are non-toxic substances. Because there is no excess or deficiency of electrons, carbene molecules are electrically neutral (nonionic).
2. How can we identify single and triplet carbene?
Ans. To determine whether a carbene is a singlet or a triplet, we must examine its connectivity and geometry. A singlet is a carbene with two atoms connected by a double bond. A triplet is a carbene with three atoms connected by single bonds. The central atom's hybridisation can also be used to identify the carbene. It is a singlet if the central atom is sp hybridised, and a triplet if the central atom is sp2 hybridised.
Conclusion
Carbenes belong to a class of highly reactive molecules having divalent carbon atoms. This means that carbon atoms use only two out of four bonds to interact with other atoms. They are usually formed as transient intermediates during chemical reactions. Based on the distribution of their nonbonding electrons, they can either be singlet or triplet carbenes. Singlet carbenes are those in which the nonbonding electrons are present in the same orbital and have opposite spins. Triplet carbenes are those in which the nonbonding electrons are present in different orbitals and have parallel or the same spins.
Multiple Choice Questions
1. Singlet and triplet carbene are the same in
(a) Types of hybridisation
(b) Number of unshared electron pairs
(c) Number of sigma - bonds
(d) Bond angle
Answer: C
2. What is the hybridisation of carbenes?
(a) sp2
(b) sp3
(c) sp
(d) sp3d
Answer: A
FAQs on Carbene and Its Types for JEE
1. What are the different carbene reactions?
Carbene reactions are mainly addition reactions, insertion reactions, and rearrangement reactions.
Addition Reaction: Carbenes usually react electrophilically. It adds itself to an alkene to produce cyclopropane derivatives. This is a (2+2) cycloaddition reaction.
Insertion reaction: Being highly reactive, they can insert themselves between C-H and C-C bonds to stabilise themselves.
Rearrangement Reaction: As carbenes are electron deficient transient intermediates and have an empty p-orbital, they can easily undergo rearrangement reactions with the migration of alkyl or hydrogen to form stable molecules.
2. What is the hybridisation of carbenes?
In singlet carbenes, the central carbon atom is sp2 hybridised. Of the three sp2 hybrid orbitals, two are used in the formation of two single bonds, with the monovalent atoms attached to the carbon. The third sp2 orbital contains the unshared pair of electrons, while the unhybridized p-orbital is empty. This electron pair has opposite spins.
In triplet carbenes, the central carbon atom is sp hybridised. The two sp hybrid orbitals are used in the formation of single bonds with the two monovalent atoms attached to the carbon. The two unhybridized p-orbitals have only one unshared electron each. These electrons have parallel spins.
3. Why is carbene majorly an electrophile?
Carbenes are predominantly electrophilic in nature. This is because the carbon in carbenes does not fulfil the octet rule. The carbon has two bonds and a lone pair of electrons. That means it has only 6 electrons, out of which 2 electrons are in the inner shell and do not participate in bonding. Since it has only 6 electrons and not 8 electrons, it can easily act as an electrophile. On the other hand, due to the lone pair of electrons, it still possesses a small degree of nucleophilicity too.
4. What is the stability order of carbenes?
In the presence of a singlet carbene, the stability order is $${{C}{F}_{2}}$$ >$${{C}{Cl}_{2}}$$ >$${{C}{Br}_{2}}$$ >$${{CI}_{2}}$$. However, when a triplet carbene is present, only inductive will determine carbene stability because there is no vacant orbital present and thus no back bonding. As a result, the order of stability is $${{C}{F}_{2}}$$<$${{C}{Cl}_{2}}$$<$${{C}{Br}_{2}}$$<$${{CI}_{2}}$$. Also, because back bonding with vacant orbitals is not available in CH2, singlet carbenes should be more stable than triplets, so the hunds rule should be applied here.

































