




Introduction to Clemmensen and Wolff Kishner Reduction
Park Davis was the first to report the Clemmensen reaction in 1913. It is the reduction of carbonyl groups (in aldehyde and ketone) to methylene groups. Clemmensen reduction is a reaction performed with zinc amalgam and hydrochloric acid. The Clemmensen reduction is particularly effective at reducing aryl-alkyl ketones, such as those formed in a Friedel-Crafts acylation.
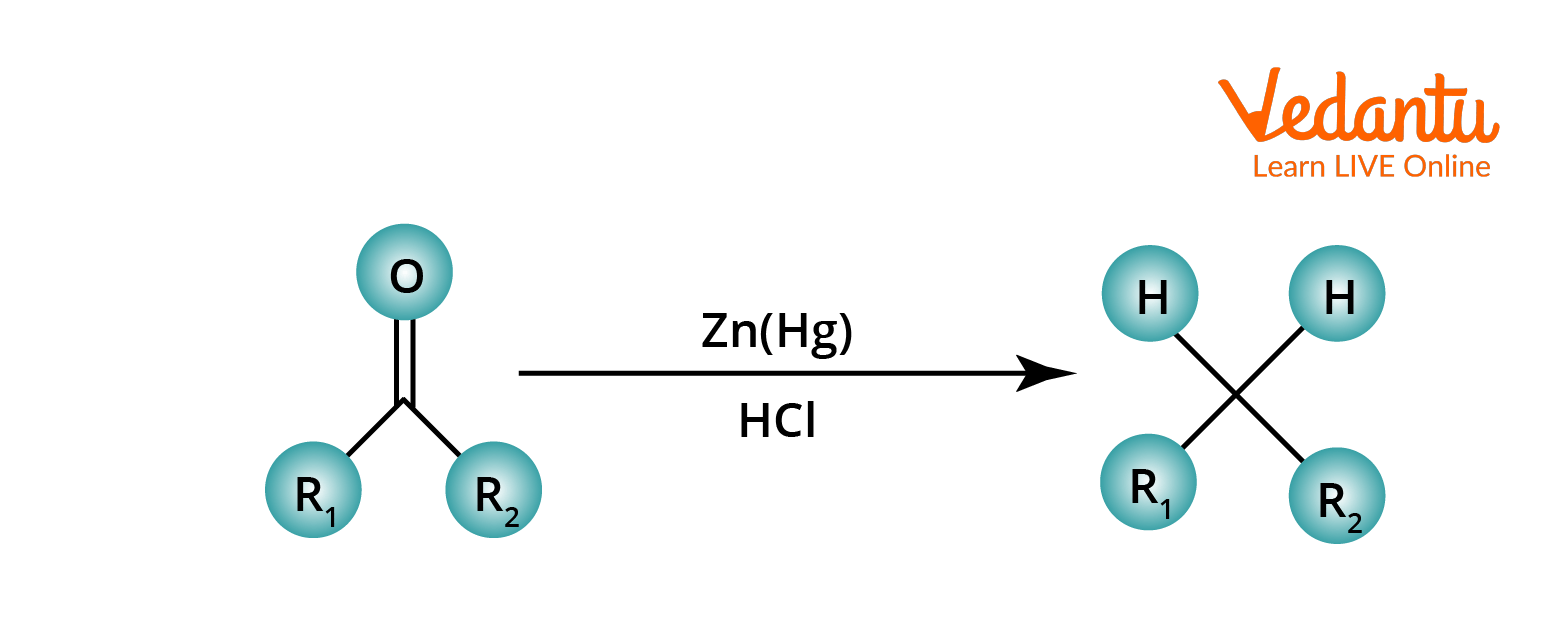
General Reaction Clemmensen Reduction
N. Kishner and L. Wolff independently discovered the Wolff–Kishner reduction in 1911 and 1912. The Wolff–Kishner reduction is an organic chemistry reaction that converts carbonyl functionalities into methylene groups. The Wolff-Kishner reduction uses hydrazine, base, and thermal conditions to convert an aldehyde or ketone to an alkane. Since the Wolff Kishner reaction needs extremely basic conditions, it is incompatible with base-sensitive substrates.
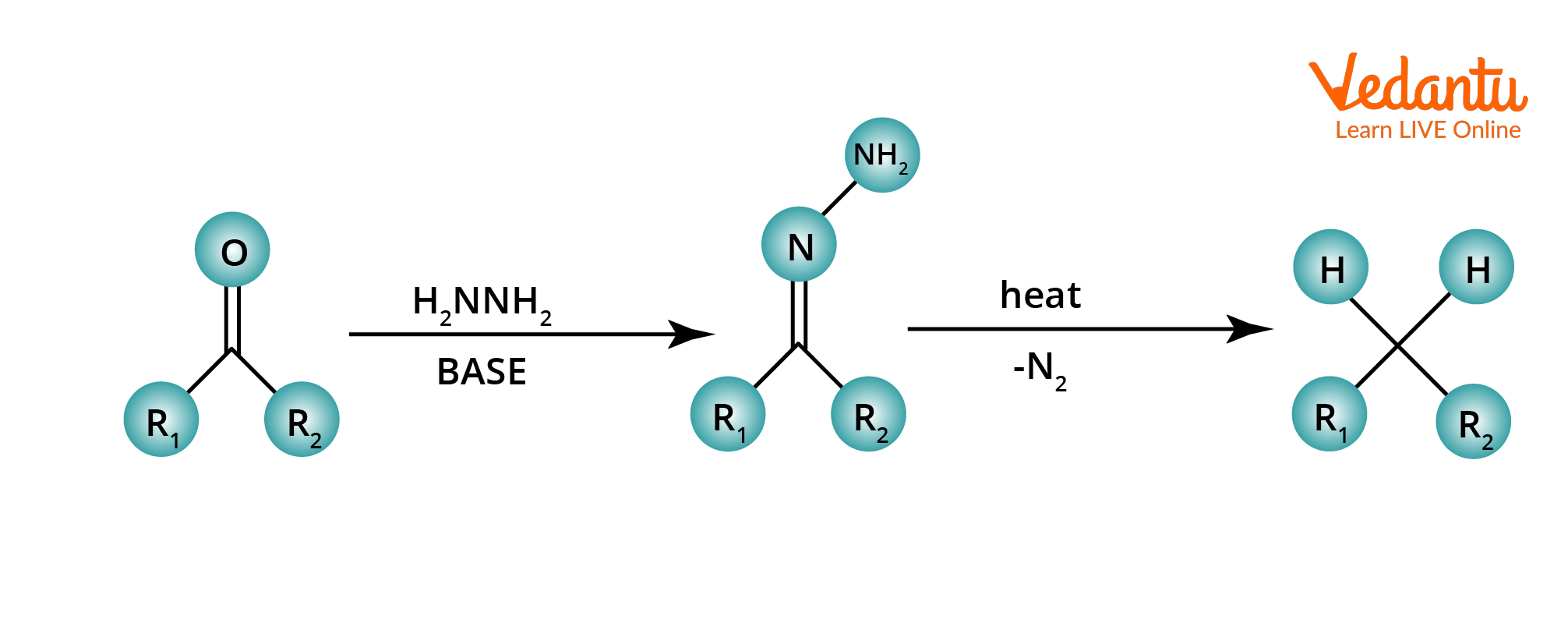
General Reaction Clemmensen Reduction
Clemmensen and Wolff Kishner Reduction
The Clemmensen reduction is most commonly used to convert acyl benzenes (from Friedel-Crafts acylation) to alkylbenzenes, but it can also be used to convert other ketones or aldehydes that are not acid sensitive. The carbonyl compound is heated with an excess of amalgamated zinc and concentrated hydrochloric acid (HCl). The actual reduction takes place on the zinc's surface via a complex mechanism.
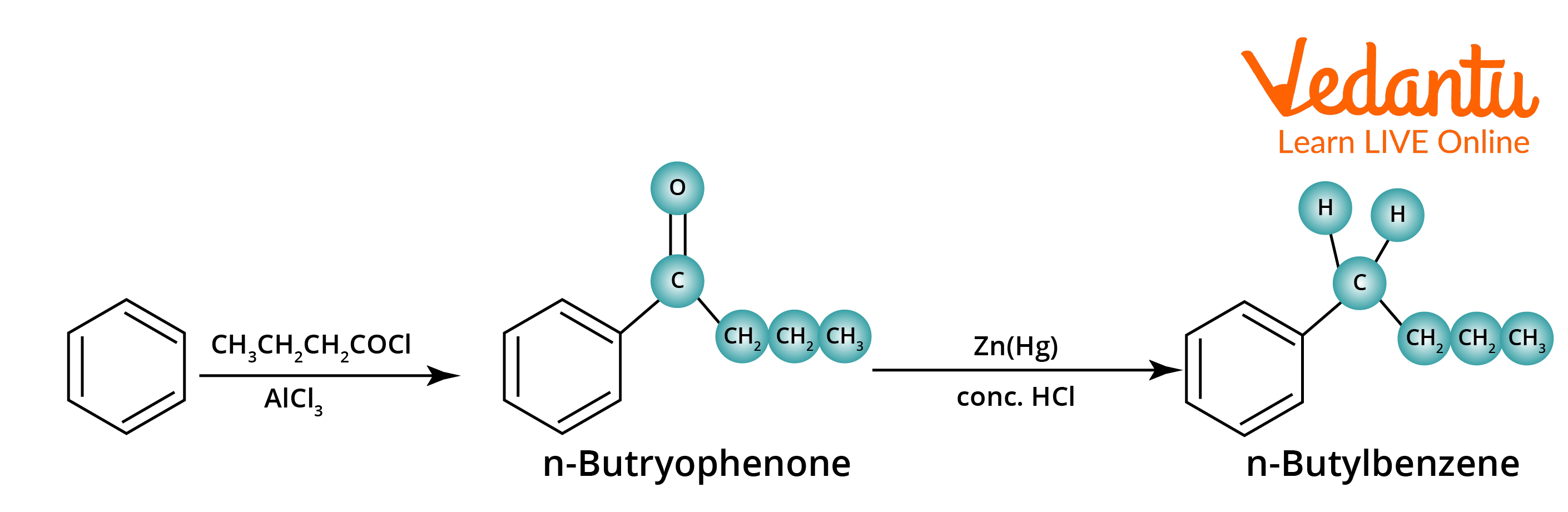
Clemmensen Reduction
Compounds that cannot withstand hot acid treatment can be deoxygenated using the Wolff–Kishner reduction. The ketone or aldehyde is converted to hydrazone by heating it with hydrazine (NH2NH2) and a strong base like KOH. To facilitate the high temperature (140-200°C) required in the second step, ethylene glycol, diethylene glycol, or another high-boiling solvent is used.
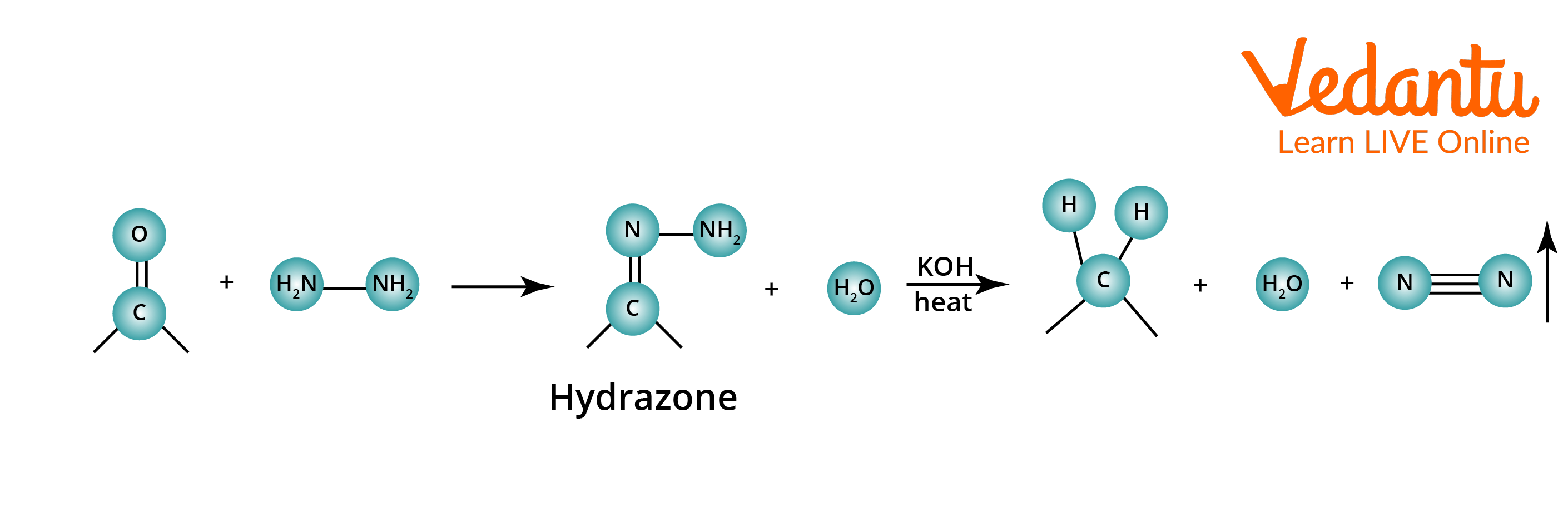
Wolff–Kishner Reduction
Difference between Clemmensen Reduction and Birch Reduction
Wolff Kishner Reduction Examples
The following are the examples of Wolff Kishner Reduction:
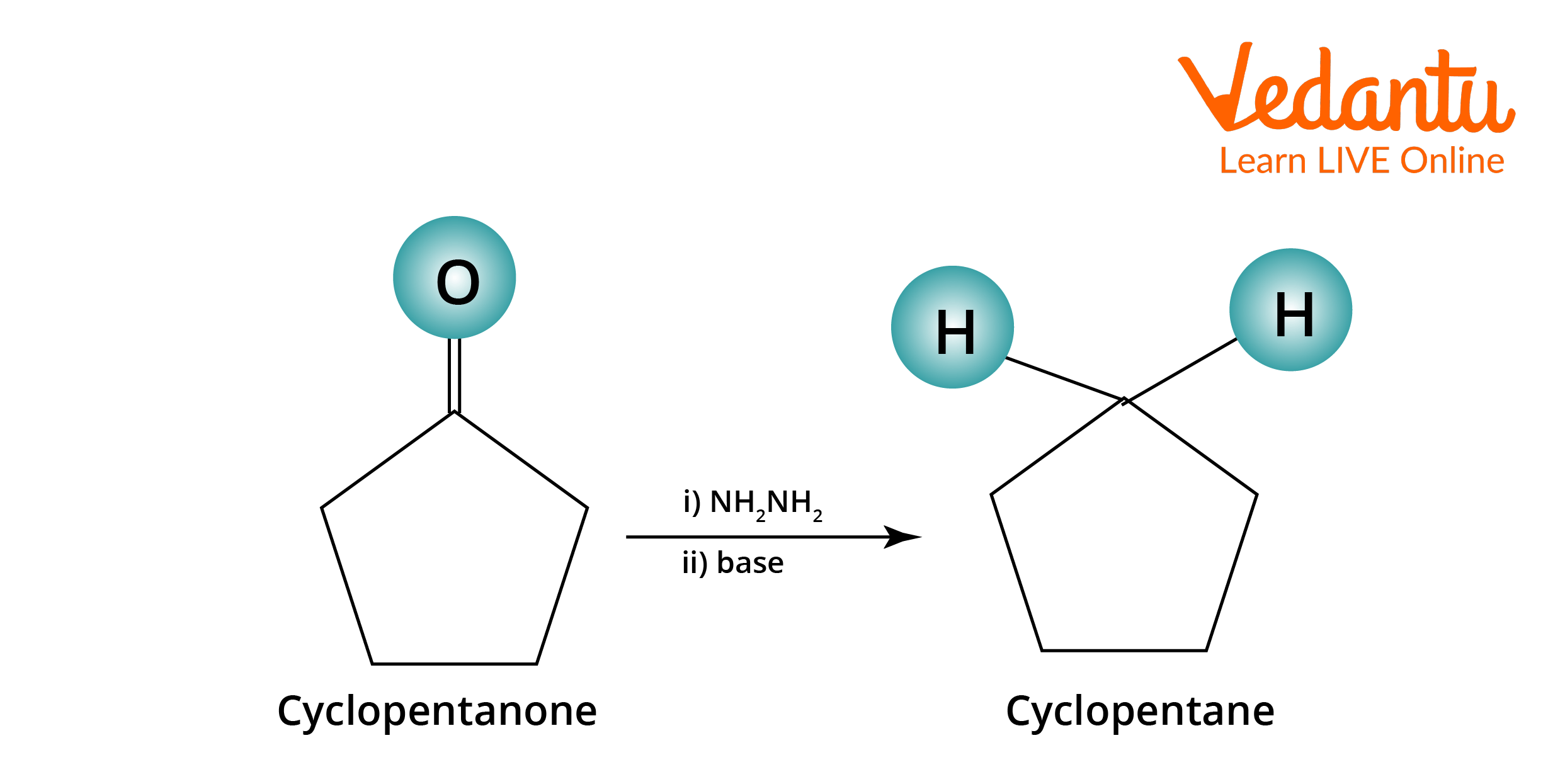
Wolff–Kishner Reduction
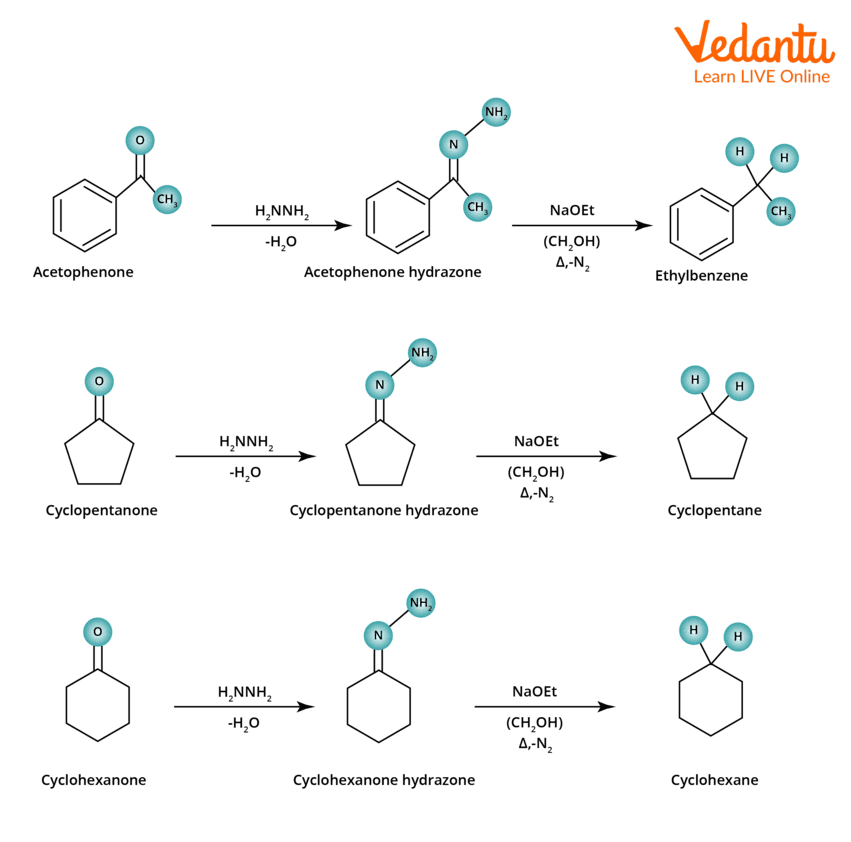
Wolff–Kishner Reduction
Wolff Kishner Reduction Mechanism
Step 1: When ketone reacts with hydrazine, hydrazone is formed, followed by a series of proton transfer steps and the expulsion of water.
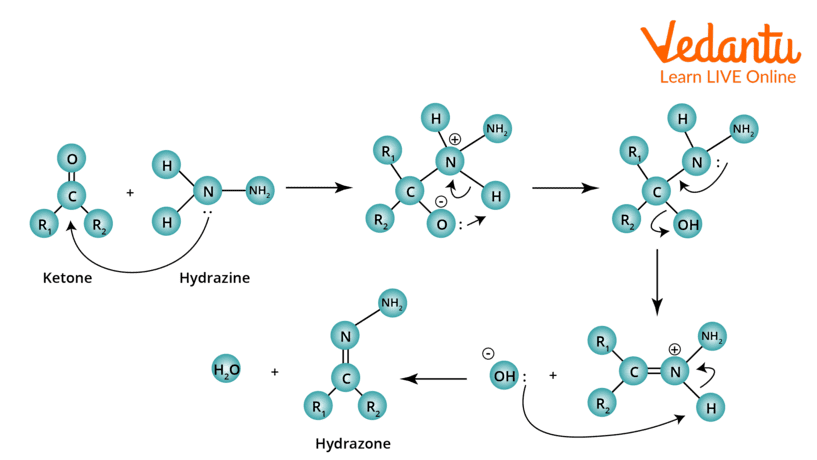
Wolff–Kishner Reduction Mechanism
Step 2: By using a powerful base to deprotonate the terminal nitrogen, a double bond is created with the nearby nitrogen to create a carbanion.
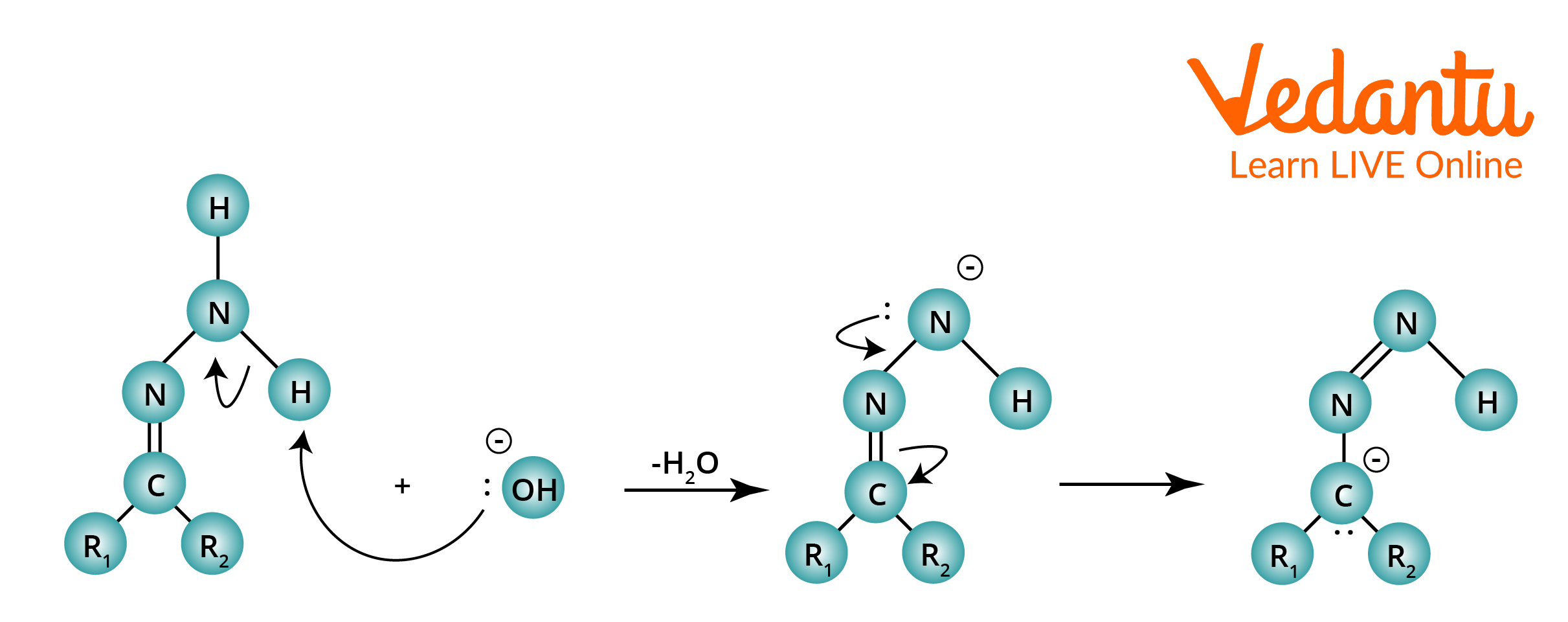
Wolff–Kishner Reduction Mechanism
Step 3: A water molecule protonates the carbon, which results in the desired alkane. This is followed by the deprotonation of the terminal nitrogen and the release of nitrogen gas.
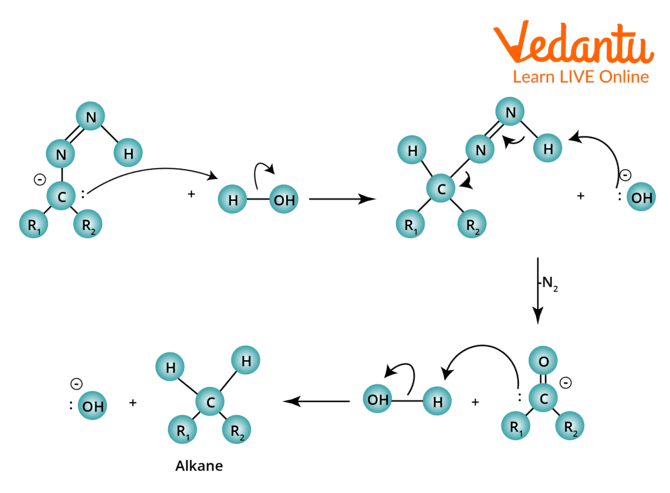
Wolff–Kishner Reduction Mechanism
Clemmensen Reduction Examples
The following are examples of Clemmensen reduction.
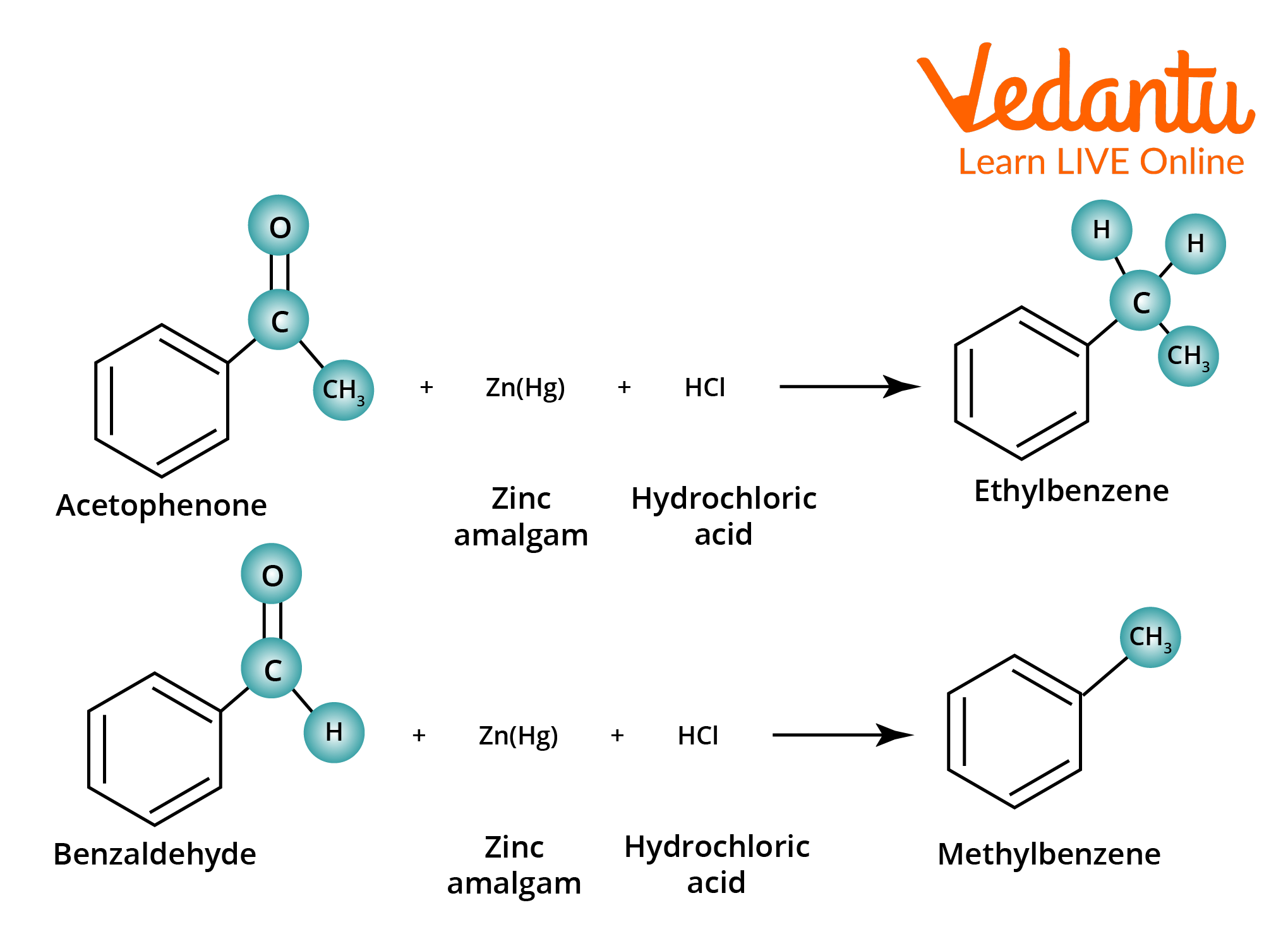
Clemmensen Reduction
Clemmensen Reduction Mechanism
Alcohols are not assumed to be intermediates in this reaction since they do not produce alkanes when subjected to the same reaction conditions. The reaction has been explained by two separate mechanisms. One process uses carbanion, in which zinc directly attacks the protonated carbon, and the other uses zinc-carbenoid, a radical reaction.
Below is a diagram of the carbanionic mechanism:
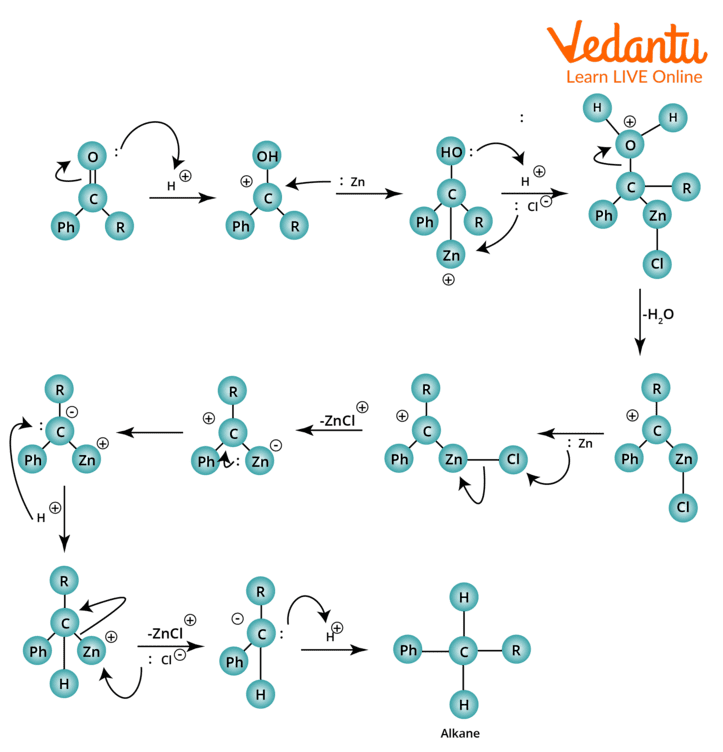
Clemmensen Reduction
Conclusion
The Clemmensen reduction is a procedure that turns aldehydes or ketones into alkanes using hydrochloric acid and zinc amalgam. Particularly well suited for this method are aryl-alkyl ketones reduced in Friedel-Crafts acylation. The finest outcomes come from the reaction when cyclic ketones or aliphatic chemicals are reduced with zinc metal. Clemmensen reduction is inappropriate for substrates that are acid sensitive, since it interacts in highly acidic environments.
Wolff-Kishner reduction occurs when the carbonyl molecule condenses with hydrazine to form hydrazone, which is then subjected to base treatment to cause carbon to be reduced along with hydrazine to be oxidised to gaseous nitrogen, and the corresponding alkane is created. Wolff-Kishner reduction is inappropriate for base-sensitive substrates since it responds to strongly basic conditions.
FAQs on Clemmensen and Wolff Kishner Reduction with Examples for JEE
1. What are the limitations of Wolff-Kishner reduction and Clemmensen reduction?
There are two important limitations to the Wolff-Kishner reduction:
It demands high temperatures.
It cannot be combined with sterically hindered ketones.
Two significant limitations of the Clemmensen reduction are:
Purely aromatic ketones do not produce adequate results. Pinacols and resinous goods frequently rule the market.
By adding a solvent that is miscible with liquid HCl, such as EtOH, CH3COOH, or dioxane, the reduction of ketonic compounds with high molecular weight and very low solubility is facilitated.
Keto acids produce poorer yields when EtOH is present.
2. What are the applications of Wolff-Kishner reduction and Clemmensen reduction?
Wolff-Kishner reduction applications are:
The transformation of a carbonyl group into a methylene group has frequently exploited this reaction.
It plays a significant role in the production of polycyclic aromatics and aromatics with unbranched side chains.
The aliphatic and mixed aliphatic-aromatic carbonyl compounds are reduced as a result of this reaction.
Clemmensen reduction applications:
Particularly for the multiwalled carbon nanotubes, this reaction has a very broad range of applications in chemical synthesis.
For the synthesis of a functionalized imidazole cation and other processes, the Wolff–Kishner reduction has also been applied on a kilogramme scale.
3. Can Clemmensen reduction reduce carboxylic acids and alcohols?
The Clemmensen reduction cannot reduce the carboxylic acid group (-COOH), but the Wolff-Kishner reduction, which involves heating the carboxylic acid after soda lime (NaOH + CaO) treatment, can. For reducing acid-sensitive substrates like this one, Wolff-Kishner reduction is appropriate.
The Clemmensen reduction can also not reduce alcohols because no alcohols are created during the reaction. The Clemmensen reaction is a common way to make alkanes, and the Clemmensen reduction is the only way to turn aldehydes and ketones into hydrocarbons such as alkanes, alkenes, and alkynes.

































