




What are Conformers?
Conformers are also known as conformational isomers. Isomers are compounds that have the same chemical formula but different structures. Conformational isomers come under the category of stereochemical isomers. Stereoisomers are those which have the same chemical formula and bond connectivity but differ in their spatial arrangement (arrangement of molecules in space).
Thus the study of conformers is important to know the mechanism and orientation of molecules in many chemical reactions.
Conformers of Alkanes
Conformers are structures that differ in rotation about a single bond. Alkanes are best to show the concept of conformation. Alkanes are organic compounds in which the connectivity of atoms is only through a single bond. Methane, ethane, and propane are some examples of alkanes. But out of this, methane will not show any conformers because, in order to show a conformer, there should be at least one C-C single bond. There is only one carbon in methane, and all other bonds are connected to hydrogens. Thus, it can have only one type of arrangement in space.
Which Formula is Used to Represent Conformations in Alkanes?
The Newman projection is a type of projection formula used to represent generally all types of conformers. In the Newman projection, we take two adjacent carbon atoms to represent its front-to-back view where the front carbon (proximal) is represented by a dot and the back carbon (distal) is represented by a circle. The image of the Newman projection of a simple alkane with only one bond is shown below.

Newman projection of alkane
In some cases, the Sawhorse projection will also be used to represent conformations. Here the adjacent two carbon atom’s bond is represented as a diagonal in which the lower end depicts the front and the upper end depicts the back or rear carbon. All other 3 bonds from the carbon atom are drawn as shown below, according to the type of conformer (staggered or eclipsed).

Sawhorse projection
Conformational Isomers Examples
The best examples to show conformational isomers are ethane, propane, and butane, of which ethane and propane are the simplest ones because it has only two conformational isomers named eclipsed and staggered. The details of the conformational isomers of ethane, propane, and butane are given below.
Conformers of Ethane
Ethane has two extreme conformers that are staggered and eclipsed. Conformers are generally represented by the Newman projection. Two conformers of ethane are shown below.
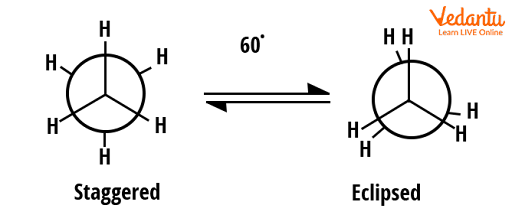
Conformers of ethane
In the above diagram, two conformers are shown; the one that has high energy is eclipsed and the one that has less energy is staggered (12 kJ/mol difference). Thus, the most stable conformer in these two will be staggered. Both can be interconverted by a rotation of 60o clockwise or anticlockwise direction.
Why Does the Eclipsed Conformer Have More Energy?
There are three reasons for the higher energy in an eclipsed conformer. They are
The dihedral angle in the eclipsed conformer is 0 which causes more steric hindrance in the structure since the two hydrogens are too close as shown in the above figure.
The repulsion of electrons in bonds is maximum in the case of an eclipsed form.
The C-H sigma bonding and antibonding orbitals of another carbon in the staggered form are parallel to each other. This will lead to a stable interaction between the two which is not possible in the case of eclipsed conformers.
Conformers of Propane
The rotational barrier between the eclipsed and staggered conformers of propane is slightly larger than that of ethane. The energy of eclipsed form in propane is 14 kJ/mol higher than that of the staggered conformer (whereas in ethane it was 12 kJ/mol). This is because in the case of propane a methyl group is present and in the case of ethane instead of this methyl group hydrogen is present. We know that the methyl group is bulkier than hydrogen, thus this steric hindrance will cause a slight increase in energy for eclipsed conformers of propane. It can be well understood from the figure given below.
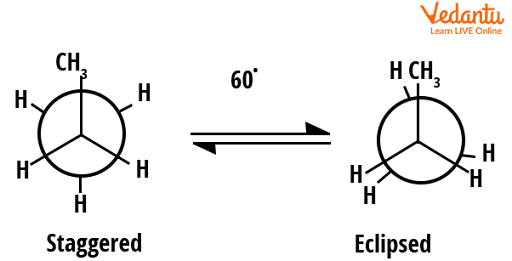
Conformers of propane
Conformers of Butane
There are an infinite number of conformations possible for butane. Out of them, only six (4 are distinct) are those which have more significance. They are syn-periplanar, synclinal or gauche, anticlinal, and anti-periplanar. In that, only 3 are conformers which have minimum potential energy (two synclinal and one anti-periplanar). The syn-periplanar (eclipsed) is the most energised conformer and that which has the least energy and is the most stable is antiperiplanar.
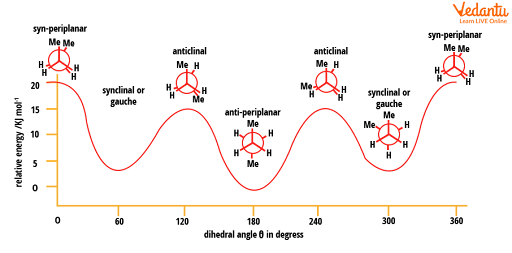
Energy diagram for conformations in butane
Conclusion
The study of conformers is very important because the arrangement of a molecule in a space can affect its involvement in reactions.
For example, the conformation and alignment of an atom can affect an elimination reaction. Products of some of the elimination reactions can only be explained on the basis of conformation differences in the reactant structure.
Thus conformations have a major role in determining the reactivity of a molecule.
FAQs on Conformers of Alkanes - Important Concepts With Examples for JEE
1. Are conformers possible for alkenes or alkynes?
Conformers differ from each other by the rotation of a single bond. If two or more bonds are present between two atoms, as in the case of alkenes or alkanes. The pi-bonds are connected by a sidewise overlap in such a way that orbitals are perpendicular to the internuclear axis. So, if we try to rotate this bond, then the pi bond will get broken.
Therefore, generally in alkenes or alkanes, conformation around the double or triple bond connection will not be possible. But if the alkenes/alkynes are not simple and have another C-C single bond in their structure, then conformers with respect to that single bond can be possible.
2. Which conformation of cyclohexane is more stable and why?
The chair conformer of cyclohexane is the most stable and least energetic among all other conformers because in a chair conformer all the C-C bonds and C-H bonds are arranged in such a manner that they can attain staggered conformation. Also, the bond angles in cyclohexane are maintained at almost 109.5o.
The other conformers of cyclohexane are boat, twist boat, and half chair. The stability of all the conformers of cyclohexane follows the order chair > twist boat > boat > half chair.

































