




What is Resonance?
Resonance describes the delocalization of electrons within molecules in the Valence Bond Theory (VBT) of bonding. It entails constructing multiple Lewis structures that, when combined, represent the molecule's entire electronic structure. When a single Lewis structure cannot fully describe the bonding, resonance structures are used.
The combination of possible resonance structures is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule. In general, molecules with multiple resonance structures are more stable than those with fewer, and some resonance structures contribute more to the molecule's stability than others – formal charges can help with this.
How to Draw Resonance Structures?
Before drawing resonance structure, we should know about certain important rules for drawing resonance structure. Only canonical or contributing structures that follow the following rules contribute to resonance.
Atom positions should be consistent across all the contributing structures. They should only differ in electron positions.
The number of unpaired electrons in all contributing structures must be the same.
The energy of contributing structures should be roughly equal.
As an example, consider carbonate ions. What is the possible number of resonating structures of carbonate ions? In a carbonate ion, each atom has an octet of electrons. There are single bonds between two carbon-oxygen atoms and one double bond between carbon and oxygen atoms in this structure. As a result, the first two C-O bonds should be distinct from the third C-O bond.
However, in experiments, all three bond lengths are equal, and the bonds are intermediate between single and double bonds. This means that the above Lewis structure fails to account for the experimental findings. To solve the problem, the CO can be represented as a resonance hybrid of the structures listed below.
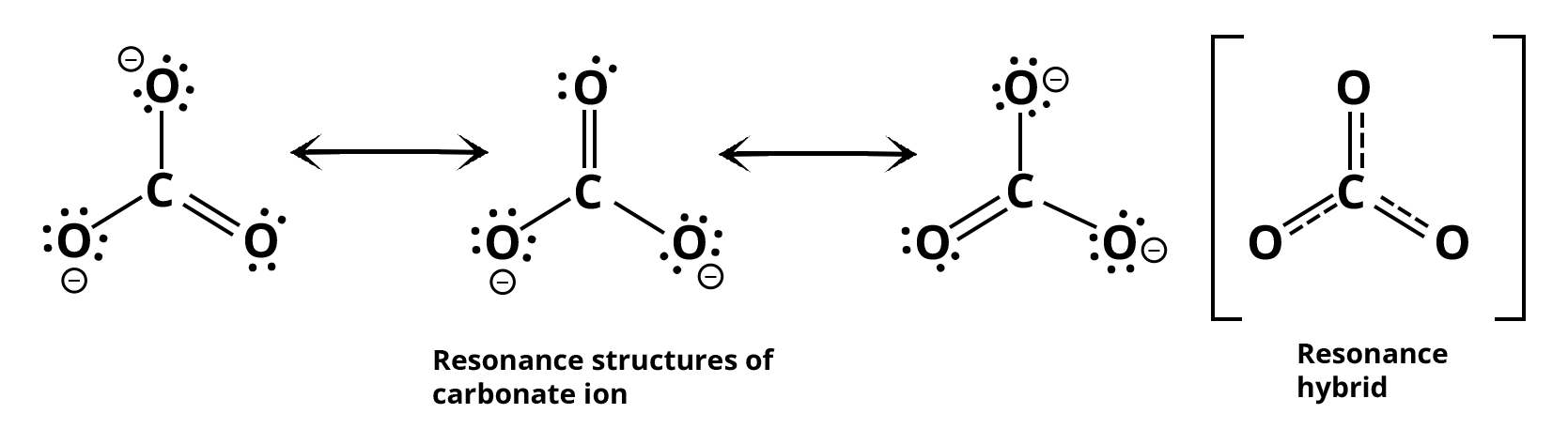
Resonance Structure of Carbonate Ions
Resonance Structure of Benzene
The possible resonating structure of benzene can be represented by a resonance hybrid of the following Kekule Lewis structure.
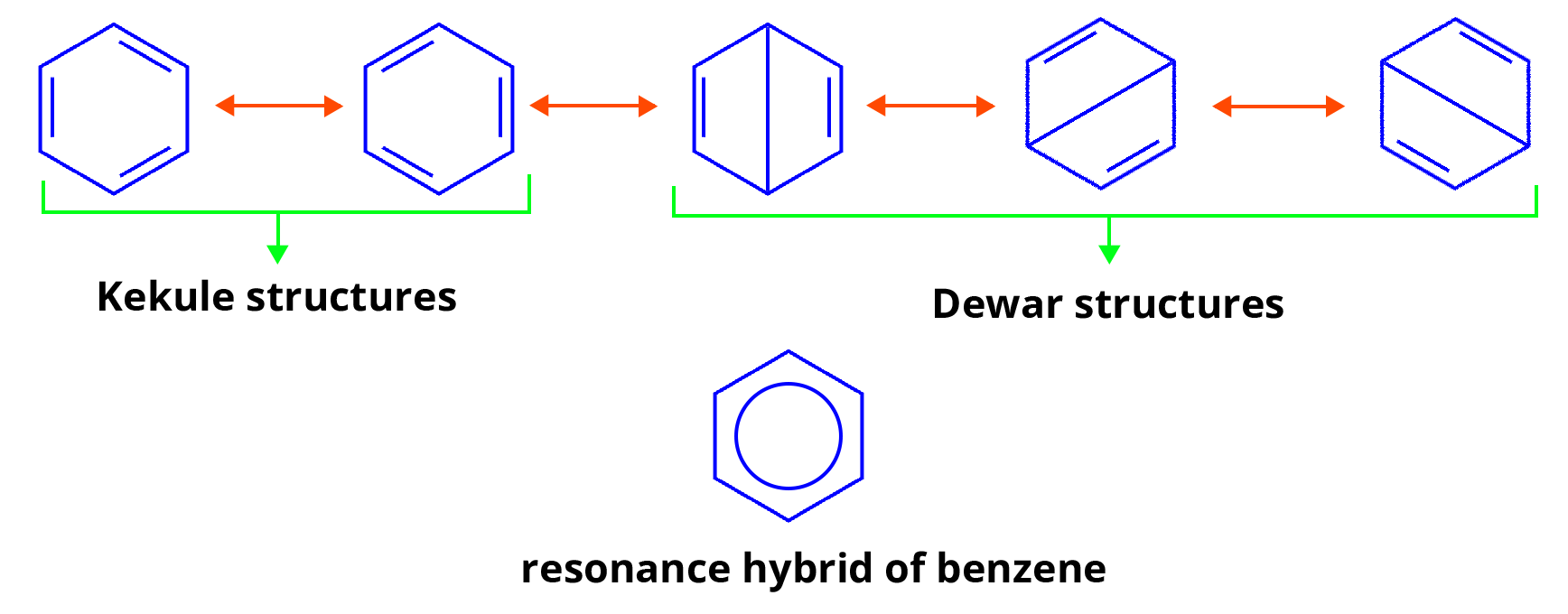
Resonance Structure of Benzene
Any of these two Kekule structures cannot explain all of the benzene's properties. A benzene molecule should have three carbon-carbon single bonds of 1.54 A length and three carbon-carbon double bonds of 1.34 A length, according to these structures. However, it has been discovered that all six carbon-carbon bonds in benzene are equal (1.39 A). This implies that the actual structure of benzene is a resonance hybrid of these two structures, rather than either I or II.
Aside from the two Kekule structures, the actual benzene molecule has a significant contribution from the three Dewar structures listed below. Thus, benzene can be thought of as a resonance hybrid of five structures, two Kekule structures and three Dewar structures. Each Kekule structure contributes about 19% to the resonance hybrid (real structure), while each Dewar structure contributes about 7%.
Resonance Structures of Chlorobenzene
How to draw resonance structures of chlorobenzene? In chlorobenzene, chlorine is an electron-donating group that transfers its electron toward the benzene ring. Hence, we can say that the electron pairs in chlorobenzene are conjugated with the ring's pi electrons. As a result of resonance, the C-Cl bond acquires a partial double bond character, which makes haloarene bond cleavage more difficult than chloromethane. Due to this, chlorobenzene is less reactive to nucleophilic reactions.
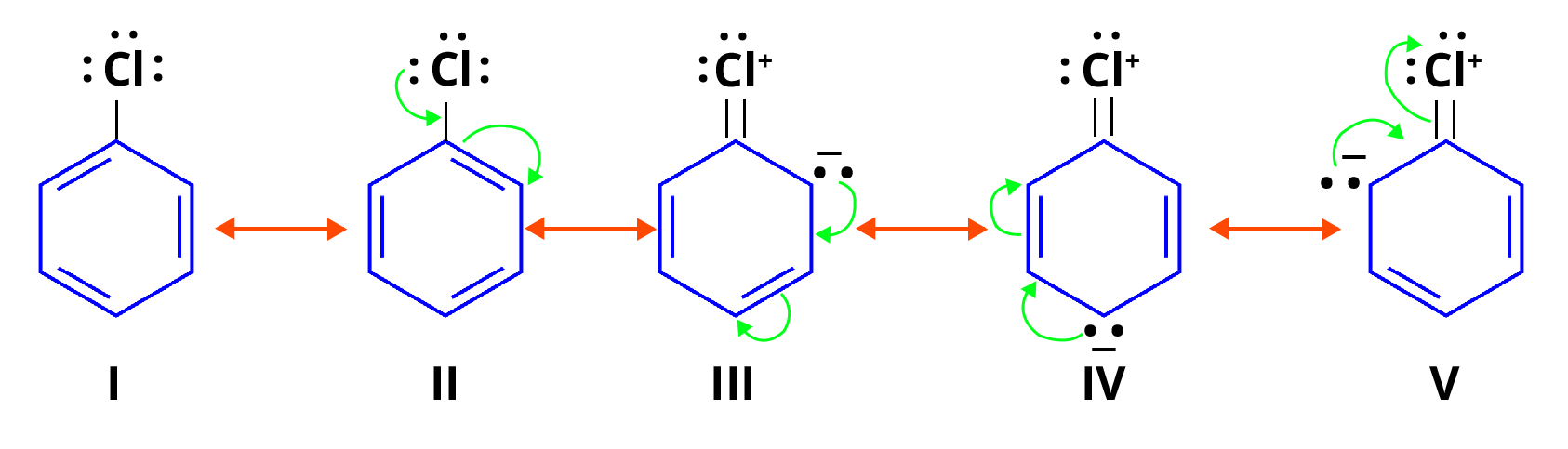
Resonance Structures of Chlorobenzene
Resonance Structures of Naphthalene
How to draw resonance structures of naphthalene? Resonance structures of naphthalene are as follows: A molecule with conjugated double bonds has alternate single and double bonds. These allow electrons to be delocalized across the entire system and thus shared by many atoms. This means that the delocalized electrons are free to move throughout the system.
We can see alternate single and double bonds in each resonance structure of a naphthalene molecule, indicating a conjugated bond system.
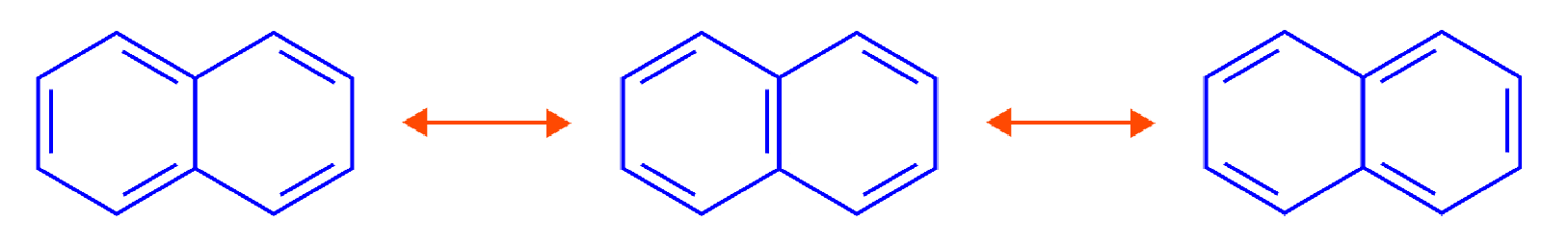
Resonance Structures of Naphthalene
Resonance Structure of CH3-O-CH2+
Draw the possible resonance structure of CH3-O-CH2+. There are two resonance structures (I) and (II) as shown below. Out of these two structures, (II) is more stable as compared to (I). This is because the stability depends on the charge present on an atom. In the second structure, the octet rule is to be followed by all-atoms i.e. carbon and oxygen. Also, the positive charge is present on the negative oxygen atom, thereby, increasing its stability.

Resonance Structures
Conclusion
For some molecules and polyatomic ions, we can draw two or more Lewis structures without changing the atom positions in the structure. Only the electron distribution varies between structures in this case. These are known as resonance structures or contributing structures. The actual molecule, on the other hand, has an intermediate structure of those possible Lewis structures. Resonance is a method of describing delocalized electrons within molecules or polyatomic ions, where the bonding cannot be expressed using a single Lewis formula.
Drawing all possible Lewis structures is the first step in drawing resonance structures. It does not have a resonance hybrid if it only has one Lewis structure.
FAQs on How to Draw Resonance Structures with Example for JEE
1. Write a short note on the characteristics of the resonating structure.
The contributing structures do not exist in reality. These are only hypothetical explanations for the molecule's properties. Only the resonance hybrid exists in reality.
Bond lengths in resonating structures become equal as a result of resonance. For instance: in ozone molecules, the 0-0 bond lengths are both equal. In benzene, all C-C bonds are equal to 0.33. The resonance hybrid has less energy and, thus, more stability than the contributing structures.
The greater the resonance and resonance energy, the greater the molecule's stability.
The concept of resonance is purely theoretical.
2. What is the formal charge and what is its significance?
The formal charge on an atom in a covalent species is the net charge the atom would have if the electrons in all of its bonds were equally shared. Alternatively, the formal charge on an atom in a covalent species is the net charge that the atom would have if all bonds to it were nonpolar covalent bonds. Use the following formula to calculate the formal charge on a given atom in a covalent species.
FC = V-N-(B/2)
FC = formal charge
V = number of valence electrons
N = number of nonbonding valence electrons
B = total number of electrons shared in bonds
























