




Step-by-Step Guide to Finding Group and Period from Electronic Configuration
Understanding how to find the group and period of an element is fundamental for JEE Main success. These classifications help students quickly analyze chemical trends and solve periodic table problems, especially when asked about electronic configurations or atomic properties. The group of an element tells you about the number of valence electrons and its main chemical behavior, while the period reveals the number of electron shells. Precise placement is vital for topics like atomic structure, block identification, and periodicity in properties.
In the modern periodic table, a group is a vertical column, whereas a period is a horizontal row. There are 18 groups and 7 periods. Groups determine similar valence shell configurations, often resulting in similar chemical and physical properties. The period number indicates how many electron shells (energy levels) are filled for that particular element. A quick look at the Periodic Table with Group Numbers and Period Numbers provides a clear visualization of these key classifications.
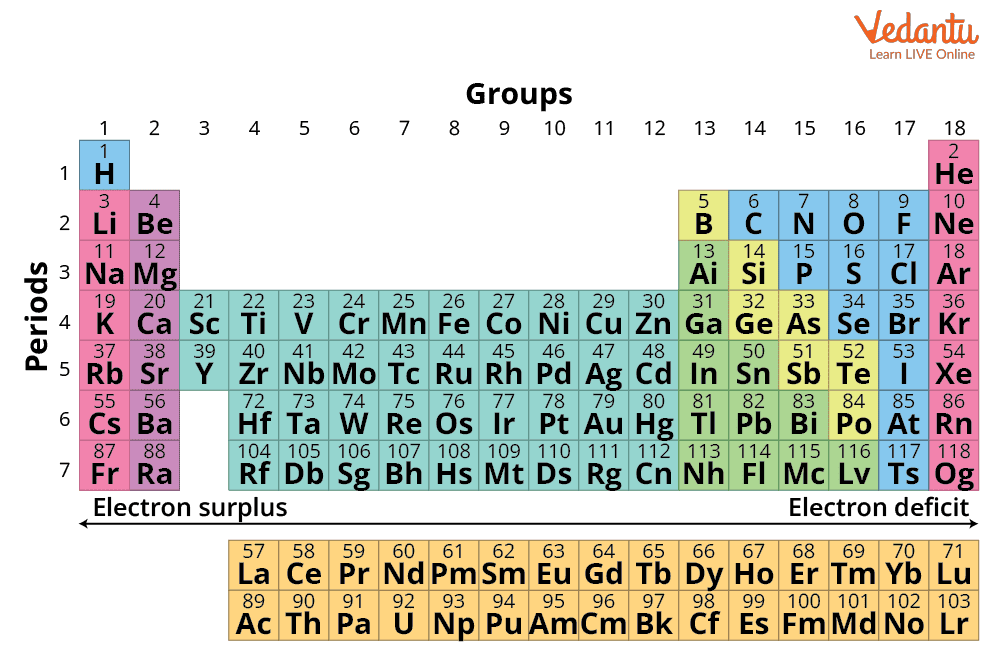
For JEE-level questions, the fastest way to determine an element’s group and period is by using its electronic configuration. This method works for all s-, p-, d-, and f-block elements, but some blocks have their own shortcuts and exceptions. Follow a systematic approach for speed and accuracy in competitive exams.
How to Find the Group and Period of an Element Step by Step
- Write the electronic configuration of the element using the atomic number.
- Count the total number of shells with electrons (principal quantum number n) to get the period.
- Find the number of electrons in the outermost shell (valence electrons) to assign the group for s- and p-blocks.
- For p-block, group number = 10 + number of valence electrons.
- For d-block (transition elements), add number of (n–1)d and ns electrons for group identification.
- Check which orbital is being filled last to know the block of the element.
Let’s apply this for a sample question: “Determine group and period of atomic number 34 (selenium).” Electronic configuration: [Ar] 3d10 4s2 4p4. Number of shells = 4 (so period = 4). Valence electrons = 6 (4s2 4p4). Selenium is a p-block element, so group = 10 + 6 = 16. Final answer: period = 4, group = 16.
Always check for the block:
- s-block: Groups 1–2; period = last shell with electrons.
- p-block: Groups 13–18; group = 10 + valence electrons.
- d-block: Groups 3–12; count (n–1)d plus ns electrons for group.
- f-block: Included in periods 6–7 as lanthanides and actinides.
Special Cases: d-Block and f-Block Rules
Transition and inner transition elements require careful counting of electrons. For d-block, group number = (n–1)d electrons + ns electrons. Example: Fe (atomic number 26) configuration is [Ar] 3d6 4s2. So, group = 6 (3d) + 2 (4s) = 8.
| Element Type | How to Find Group | Period Rule |
|---|---|---|
| s-block | Group = valence electrons (1–2) | Period = number of shells |
| p-block | Group = 10 + valence electrons | Period = number of shells |
| d-block | Group = (n–1)d + ns electrons | Period = outermost shell |
| f-block | No group number assigned, period 6 or 7 | 6 (lanthanides), 7 (actinides) |
For inner transition elements, the period is easy (6 or 7), but they sit off the main group numbering. For JEE, always note block and period if group is undefined.
Group and Period Number: Chemical Properties Link
Elements in the same group display similar chemical reactivity because of matching valence shell electron counts. For example, group 1 elements (alkali metals) are highly reactive metals due to their single valence electron. The period number indicates how many shells the atom has, so down a group, size and metallic character increase. This insight is crucial in predicting trends such as ionization energy and electronegativity, explained in greater depth at Periodic Trends.
Solved Examples: JEE Main Style
Let’s reinforce exam skills with selected solved examples on how to find the group and period of an element:
- Atomic number 17 (chlorine): [Ne] 3s2 3p5; period = 3, group = 10 + 7 = 17.
- Atomic number 20 (calcium): [Ar] 4s2; period = 4, group = 2 (s-block).
- Atomic number 42 (molybdenum): [Kr] 4d5 5s1; d-block, group = 5 + 1 = 6, period = 5.
- Atomic number 64 (gadolinium): [Xe] 4f7 5d1 6s2; f-block, period = 6.
For plenty of JEE-style numerical drills, try Classification of Elements and Periodicity Practice Paper or Mock Test 1.
Common Errors and Revision Pro-Tips
- Missing the correct block—p-block starts at group 13, not group 11.
- Forgetting to add d and s electrons for d-block groups.
- Mistaking period for principal quantum number in some configurations.
- Overlooking exceptions (e.g., chromium and copper group assignments).
- Not checking noble gas shortcuts in electronic configuration.
- Equating period with highest subshell (must be the highest value of n with at least one electron).
Quick Revision and Essential Resources
- Memorize main group and period numbers from a blank periodic table—download from Modern Periodic Table.
- Keep a summary of group and block rules ready for last-minute revision.
- Practice writing complete electronic configurations for first 30 elements—role in rapid group/period determination is critical.
- Review d- and f-block rules and exceptions.
- Use Vedantu’s periodic table memory aids for quick group/period reference during practice tests.
Mastering how to find the group and period of an element ensures you answer at least 2–3 questions correctly in every JEE Main Chemistry paper. Mark the formulae, practice consistently, and check your answers with Vedantu’s expert-reviewed solutions and cheat-sheets.
FAQs on How to Find the Group and Period of an Element
1. How do you know what group and period an element is in?
To determine the group and period of an element, analyze its electronic configuration:
• Write the electronic configuration of the element.
• Period number = number of electron shells (energy levels) present.
• Group number (for s- and p-block): For s-block, group = number of valence electrons; for p-block, group = number of valence electrons + 10.
• For d- and f-block, special group assignment rules apply.
Using these steps helps you quickly locate any element's position in the periodic table for board or JEE/NEET exams.
2. What is the group and period of atomic number 114?
Element 114 (Flerovium) lies in:
• Group 14 (p-block element)
• Period 7
This is determined by writing its electronic configuration and counting both the valence shell (period) and valence electrons (group): its outermost electrons fill the 7th shell and p-block, placing it in group 14, period 7.
3. What are the 7 periods and 18 groups in the periodic table?
The periodic table consists of 7 horizontal periods and 18 vertical groups:
• 7 periods represent energy levels/shells (rows numbered 1 to 7).
• 18 groups are the columns (numbered 1 to 18) indicating similar valence electron configurations and chemical properties.
This layout helps differentiate elements by their chemical behavior and atomic structure.
4. Where do you find a group of elements in the periodic table?
Groups are the vertical columns in the periodic table:
• There are 18 groups, numbered from 1 to 18 from left to right.
• All elements in a group share similar valence electron configuration and properties.
Use the group number to predict reactivity and chemical characteristics.
5. How do you find group and period from electronic configuration?
Finding group and period from electronic configuration involves:
1. Counting the number of shells = Period number.
2. Analyzing valence electrons:
● s-block: Group = valence electrons
● p-block: Group = valence electrons + 10
3. Identifying block (s, p, d, f) for correct group assignment.
This approach is vital for quick exam solutions and understanding periodic trends.
6. How to determine group and period of an element quickly during exams?
To quickly find an element's group and period in exams:
• Identify the highest principal quantum number (n) in its electronic configuration → this is the period.
• Count total electrons in the valence shell(s):
● s-block: group = valence electrons
● p-block: group = valence electrons + 10
• For d/f-block: Use special rules by adding d-electron count appropriately.
This stepwise shortcut saves time and ensures accuracy under exam pressure.
7. How do group and period numbers affect the properties of elements?
Group and period numbers influence an element's chemical and physical properties:
• Elements in the same group have similar valence electrons and chemical reactivity.
• Elements in the same period show gradual change in properties (metallic to non-metallic) across the row.
Understanding position helps predict periodic trends like atomic size, electronegativity, and reactivity.
8. Are there special rules for finding group numbers of d-block and f-block elements?
Yes, d- and f-block group calculation uses specific rules:
• d-block (transition) elements: Group = (number of electrons in (n-1)d) + (number of electrons in ns).
• f-block (inner transition) elements: Generally placed in period 6 or 7 and not assigned a group number in the main table.
Refer to the periodic table's structure for these exceptions in competitive exams.
9. Is electronic configuration always needed to find group and period?
Electronic configuration is the most reliable method, but:
• For familiar elements, you can use periodic trends or group/period patterns.
• For unknown elements, electronic configuration is essential.
Accurate group and period identification relies on knowing the arrangement of electrons.
10. Can two elements in the same period have different chemical behavior?
Yes, elements in the same period can show different chemical behavior:
• Moving left to right in a period, valence electrons increase, leading to a range of metallic to non-metallic properties.
• This causes differences in reactivity, bonding, and compounds formed.
Hence, period tells us about shell number, but group reveals more about specific reactivity.























