




Stepwise Nomenclature Guide for Bicyclo and Spiro Compounds with Examples
The Naming Of Bicyclo And Spiro Compound plays a crucial role in JEE Main Organic Chemistry, as these complex ring systems are often tested in exams. Understanding the IUPAC conventions for bicyclic and spirocyclic compounds lets you confidently identify, name, and differentiate their structures in competitive questions. This topic covers the special rules for numbering carbon atoms, writing names using brackets, and correctly handling substituents or heteroatoms in bridged, fused, and spiro systems.
Bicyclic and spirocyclic compounds are important structural motifs in organic chemistry. Both involve cyclic systems connected by a specific carbon arrangement, but their connectivity and naming logic differ sharply. Mastering these distinctions strengthens your grasp of JEE-relevant nomenclature and structure identification, which also supports analysis of isomerism and reaction mechanisms.
Basics of Bicyclo and Spiro Compounds
In the Naming Of Bicyclo And Spiro Compound, compounds are classified based on how two or more rings are joined. Bicyclic compounds have two rings sharing at least two carbons (called bridgeheads), while spirocyclic compounds have two rings that meet at only one common atom (the spiro atom). Both are saturated systems unless indicated; unsaturation or heteroatoms follow normal IUPAC designation.
Visualizing the framework is key—bicyclics often appear as fused or bridged structures, and spiro compounds show two rings connected at a single point. Recognizing these motifs helps avoid common errors in number assignment and ring count.
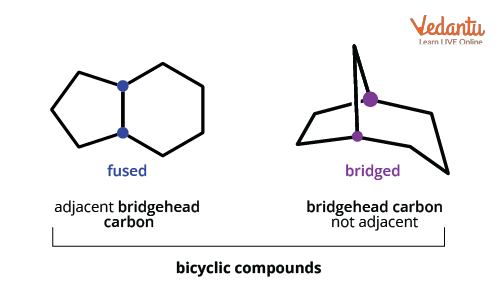
For clarity, the fused and bridged types in bicyclics have distinct connectivity. Fused has the rings joined directly, and bridged includes an extra chain (bridge) between the ring junctions.
Difference Between Bicyclo and Spiro Nomenclature
Bicyclo compounds: Two rings share two or more bridgehead carbons, with possible one or more bridges connecting them.
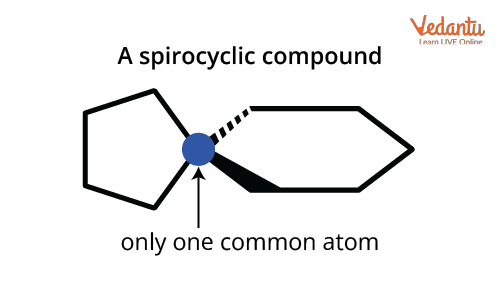
Spiro compounds: Only one carbon (the spiro atom) is common to both rings. No true bridge exists, just a shared atom. These differences affect not just naming but also physical properties and isomerism type.
| Aspect | Bicyclo Compound | Spiro Compound |
|---|---|---|
| Rings joined by | Two or more bridgehead carbons | One spiro atom (single carbon) |
| Nomenclature base | bicyclo[x.y.z] | spiro[x.y] |
| Number order in brackets | Descending | Ascending |
| Common in JEE questions? | Very | Yes |
Recognize these distinctions to solve naming, isomerism, and structure questions efficiently. For additional practice, visit Cycloalkanes and Allenes and Structural Isomerism.
IUPAC Rules for Naming Bicyclo Compounds
The rules for the Naming Of Bicyclo And Spiro Compound, specifically for bicyclics, follow a precise order:
- Count the total number of carbon atoms in the entire molecule (including all rings).
- Identify the two bridgehead carbons (shared by both rings).
- Count and list, within brackets, the number of carbons in each of the three bridges connecting the bridgeheads—listed in descending order (x.y.z).
- Name as bicyclo[x.y.z]alkane, where the base alkane reflects the total carbons.
- Start numbering from one bridgehead, proceed along the longest bridge, then the next longest, then the shortest, ending at the other bridgehead.
For example, bicyclo[2.2.1]heptane (norbornane) has 7 carbons, with bridges of 2, 2, and 1 carbon each. Note, if substituents are present, their position is given by the number assigned in the above sequence, and multiple substituents are arranged alphabetically in naming.
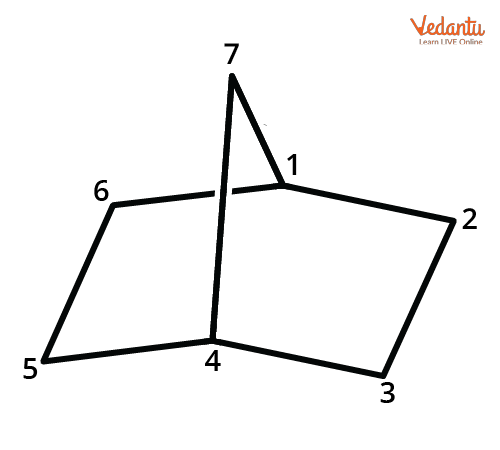
Practice examples like bicyclo[2.2.2]octane and bicyclo[3.2.1]octane to get used to the structure and number system. When bridges are zero, it means rings are simply fused (e.g., bicyclo[4.4.0]decane for decalin).
Nomenclature of Spiro Compounds
For the Naming Of Bicyclo And Spiro Compound in spirocyclic systems, the IUPAC rules are similar but with important differences:
- Count all carbon atoms in the molecule (excluding hydrogens or heteroatoms for the base name).
- Write as spiro[x.y]alkane, where x and y are the number of carbon atoms (not counting the spiro atom itself) in the two rings attached to the spiro center.
- Numbers inside the brackets are ordered in ascending value (lowest to highest), a key contrast to bicyclic systems.
- Start numbering from the smaller ring, beginning with a carbon next to the spiro atom, go around the smaller ring, return to spiro atom, then continue through the larger ring.
- Assign substituent positions based on this numbering for complete names.
For instance, spiro[4.5]decane has a spiro atom sharing two rings; one with 4 and one with 5 atoms. Numbering ensures uniqueness and alphabetical substituent order. Always avoid mistakes in bracket order—JEE often tests if you confuse ascending with descending!
Naming with Substituents and Special Cases
Most JEE Main questions focus not just on the basic Naming Of Bicyclo And Spiro Compound structures, but also on cases with alkyl, halogen, or functional substituents. Remember:
- Number the base as per the compound class (bicyclo or spiro).
- Assign the lowest possible numbers to substituents while following the correct bridge-sequence.
- If functional groups are present, follow priority rules (carboxylic acids, aldehydes, etc.).
- For heteroatoms: include their position numbers and name the heteroatom prefix before the base skeleton.
For example, in 2-bromo-4-chlorospiro[3.4]octane, ‘2-bromo’ indicates the position in the smaller ring and ‘4-chloro’ in the larger. Working extra example problems on naming and drawing such substituted structures is essential.
JEE also asks about chiral centers and isomerism in some bicyclo compounds, for example in questions on optical activity in allene, biphenyl, or certain spirocycles. Refer to Optical Isomerism in Allene, Spiro, and Biphenyl for more.
JEE Traps and Revision Tips
Be aware of classic mistakes in the Naming Of Bicyclo And Spiro Compound:
- Mixing up ascending and descending order in brackets.
- Mis-counting carbons in bridges or rings—always double-check.
- Assigning wrong ring sizes when drawing structures from names or vice versa.
- Omitting bridgehead or spiro carbons in numbering.
- Confusing spiro compounds with fused rings in tricky diagrams.
Quick revision sheets, such as those on polyfunctional compounds naming or aldehyde and ketone naming, enhance last-minute prep. Organize practice for all rules, especially with complex bridged examples.
Summary Table: Bicyclo & Spiro Naming Essentials
| Feature | Bicyclo | Spiro |
|---|---|---|
| General Format | bicyclo[x.y.z]alkane | spiro[x.y]alkane |
| Bracket Order | Descending (largest to smallest) | Ascending (smallest first) |
| Example Name | bicyclo[2.2.1]heptane | spiro[3.4]octane |
| Base Structure | Two bridgehead carbons, bridges | One spiro atom, two rings |
For a comprehensive approach, combine this summary with detailed NCERT and organic chemistry revision notes. These resources provide extra practice naming complex rings as per JEE trends.
Remember, consistent application of the Naming Of Bicyclo And Spiro Compound conventions is crucial for full marks in JEE Main. Vedantu's worked examples use up-to-date IUPAC rules and match the exam format.
- Revise common compounds: bicyclo[2.2.2]octane, spiro[4.5]decane, bicyclo[3.2.1]octane, spiro[3.4]octane.
- Practice substituent placement and bridge numbering repeatedly.
- Use JEE Main mock questions on polycyclic compounds.
- Consult Hydrocarbons and Structural Isomers and Its Types for related topics.
- Cross-check each name with a hand-drawn structure for accuracy.
By mastering the Naming Of Bicyclo And Spiro Compound and related variants—like “spirocyclic nomenclature”, “bicyclo naming”, and “rules for naming spiro compounds”—you boost accuracy and speed in JEE Main chemistry. For complete preparation material, explore other Vedantu Chemistry resources tailored to the latest syllabus.
FAQs on Naming of Bicyclo and Spiro Compounds Made Easy
1. How do you name bicyclo and spiro compounds?
To name bicyclo and spiro compounds in chemistry, follow IUPAC rules that use bracketed numbers to show how carbons are connected.
• Bicyclo compounds: Use “bicyclo[x.y.z]alkane” format, where x, y, and z are the numbers of carbon atoms in each bridge connecting the two bridgehead carbons.
• Spiro compounds: Use “spiro[x.y]alkane”, where x and y are the number of carbons on each side of the spiro (shared) carbon.
• Number the parent carbon chain to give the lowest possible numbers to the bridgehead (bicyclo) or spiro (spirocyclic) positions.
This systematic nomenclature is essential for JEE, NEET, and board exams as per the current chemistry syllabus.
2. What is the difference between a spiro and a bicyclo compound?
The main difference is their structural connection:
• Bicyclo compounds have two rings that share two or more carbon atoms (bridgeheads), forming multiple bridges.
• Spiro compounds have two rings connected through a single common atom, called the spiro carbon.
This distinction affects how they are named using IUPAC rules and is important for understanding organic nomenclature in competitive exams.
3. What is the meaning of the numbers in “bicyclo[2.2.1]heptane”?
In bicyclo[2.2.1]heptane, the numbers in the brackets represent the number of carbons in each bridge between the two bridgehead carbons.
• '2.2.1' refers to:
1. Two carbons in the first bridge
2. Two carbons in the second bridge
3. One carbon in the third bridge
• The total carbon number in the parent name (heptane) is 7, which includes all ring and bridge carbons.
This scheme is a key part of organic compound nomenclature.
4. How do you assign numbering in spiro compounds?
Numbering in spiro compounds starts at the smallest ring, continues through the spiro carbon, and ends at the larger ring:
• Begin numbering from the end of the smaller ring, moving towards the spiro atom.
• Continue numbering through the spiro atom and then around the larger ring.
• Ensure substituents receive the lowest possible locants.
This order is required by IUPAC and helps avoid confusion in exams.
5. Are there exceptions to the general naming rules for bicyclic and spiro compounds?
Yes, while most bicyclo and spiro compounds follow the standard IUPAC rules, exceptions may occur with:
• Presence of heteroatoms (atoms other than carbon)
• Use of common/trivial names for well-known compounds
• Substituent or functional group priority in complex systems
Always follow the most updated IUPAC guidelines for naming exceptions.
6. How to name bicyclic compounds with substituents?
To name bicyclic compounds with substituents:
• Identify the parent bicyclo structure and assign numbers to all carbons, starting at a bridgehead.
• Number through the longest path first, then the next longest, ending with the shortest.
• Assign the lowest possible numbers to substituents.
• Name and order the substituents as prefixes, then write the main bicyclo core name.
This approach ensures clarity and meets IUPAC standards for exam solutions.
7. What are common mistakes students make while naming spiro and bicyclo compounds?
Common mistakes in naming spiro and bicyclo compounds include:
• Incorrectly identifying bridgehead or spiro carbons.
• Numbering carbons in the wrong direction or order.
• Miscounting carbons in each bridge or ring.
• Ignoring substituent priority or breaking IUPAC locant rules.
Carefully following stepwise nomenclature prevents these errors in JEE and board exams.
8. Can a compound be both spiro and bicyclo at the same time?
No, a compound cannot be classified as both spiro and bicyclo simultaneously because these terms describe mutually exclusive structural features:
• Spiro compounds share one atom between two rings.
• Bicyclo compounds share at least two bridgehead atoms between rings.
The IUPAC nomenclature assigns one specific type based on structure.
9. Does the presence of heteroatoms change the naming rules for bicyclic or spiro systems?
Yes, when heteroatoms (like O, N, S) are present in spiro or bicyclic compounds:
• Use appropriate prefixes such as “oxa”, “aza”, or “thia” to denote their positions.
• Numbering must include heteroatoms while following IUPAC locant priority.
• The core structure and bracket format remain the same.
This is vital for accurate naming in advanced questions.
10. How to download worksheets or PDFs for practice on naming of bicyclo and spiro compounds?
You can access practice worksheets and revision PDFs for naming of bicyclo and spiro compounds from reputable JEE/NEET preparation websites or your coaching institute.
• Check chemistry resource sections for topic-specific worksheets.
• Look for solved examples and stepwise nomenclature problems.
• Use these for rapid revision before exams, ensuring all latest syllabus points are covered.























