




What are Atoms and Molecules?
The "fundamental building blocks of matter" are described as atoms.
It is the lowest component of matter that possesses chemical elemental characteristics. Atoms don't exist on their own; instead, they unite to create ions and molecules, which then join together to create the matter that we can see, feel, and touch. Atoms are considerably too small to be observed, tests to understand their structure and behavior must be carried out using a vast number of them. We can try to create a hypothetical model of an atom that acts like the real atom using the findings from these studies.
A single or more atoms are joined together by covalent (chemical) bonds to form molecules. Atoms can be represented by spherical shapes, each of which has a nucleus at the center (contains protons and neutrons), surrounded by one or more concentric circles, which represent the "shells'' or "levels" in which the electrons surrounding the atom's nucleus are located, and markings indicating the electron at each level. The tiniest unit into which a substance can be further divided is a molecule.
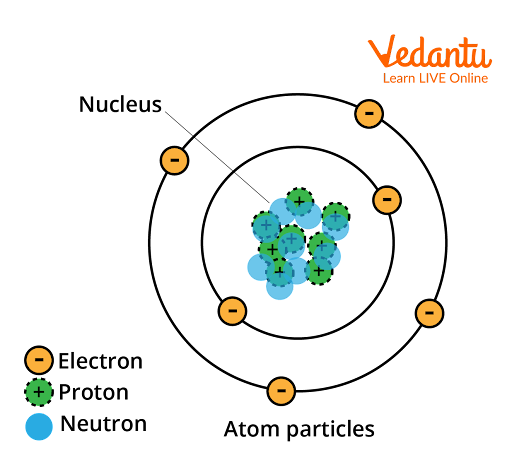
Image: Atom Particle
Atomic mass
The total mass of the nucleons in an atom's nucleus is known as its atomic mass. Either a proton or a neutron makes up a nucleon. Therefore, the combined mass of the protons and neutrons in the nucleus is what is known as the atomic mass. Although there are electrons in atoms as well, their mass is not taken into account in the calculations because electrons are much smaller and have a much lower mass than protons and neutrons.
In contrast to relative atomic mass, we determine the mass of every single atom here without estimating an average value. As a result, the atomic masses of the various isotopes have distinct values. This is because various isotopes of the same element have varying numbers of nucleons.
Hence, the atomic mass of an element can be found out by simply adding the mass of protons and neutrons in that element. The unit of atomic mass is atomic mass units (amu).
Atomic Mass = Mass of protons + Mass of neutrons.
Relative Atomic Mass
A dimensionless physical quantity known as relative atomic mass (symbol: Ar) or atomic weight is defined as the ratio of the average mass of atoms of a chemical element in a particular sample to the atomic mass constant. The definition of the atomic mass constant (symbol: mu) is $\dfrac{1}{12}^{th}$ of the mass of an atom of carbon-12. Since the two components of the ratio are masses, the final value has no dimensions, which is why it is referred to as a relative value.
The weighted arithmetic mean of the masses of the individual atoms (including their isotopes) that are present in a sample determines the relative atomic mass of a given element.
The relative atomic mass values obtained from numerous different samples are applied to produce the more prevalent and precise quantity known as standard atomic weight (Ar, standard). According to some interpretations, it refers to the predicted range of the relative atomic mass values for the atoms of a specific element from all terrestrial sources, with the sources all coming from Earth.
Hence, we define Relative atomic mass (RAM or Ar) as the weighted average of an element's isotope masses in relation to the mass of a carbon-12 atom.
For example, the relative atomic mass of oxygen is 15.999 u, relative atomic mass of sodium is 22.989769 u. Since relative atomic mass is a ratio, there is no unit of relative atomic mass.
How to Calculate Relative Atomic Mass?
Instead of using an individual mass value, relative atomic mass must be employed to accomplish precise chemical computations. As a result, relative atomic mass accounts for all of an element's stable isotopes that exist in nature. The relative atomic mass, or average mass, can be computed from the composition's percentage easily ( percent abundance). The formula for relative atomic mass is
$A_{r}=\dfrac{\sum \text { isotopic mass } \times \text { total isotope abundance }}{100}$
For example Chlorine has two isotopes, 75 % chlorine – 35 ( 75 %)and chlorine – 37 (25%)
The relative atomic mass of chlorine is $A_{r}=\dfrac{35 \times 75+37 \times 25}{100}=35.5$
Conclusion
The "fundamental building blocks of matter" are described as atoms.
It is the lowest component of matter that possesses chemical elemental characteristics. Single or more atoms are joined together by covalent (chemical) bonds to form molecules. Atoms can be represented by circle shapes, each of which has a nucleus at the center.Relative atomic mass (RAM or Ar) is the weighted average of an element's isotopes' masses in relation to the mass of a carbon-12 atom.
For example, the relative atomic mass of oxygen is 15.999 u. Here we discussed how to calculate the relative atomic mass of elements, and the relative atomic mass formula for calculating it was also discussed. It is a unitless quantity as it is a ratio.
FAQs on Relative Atomic Mass and Its Calculation for JEE
1. Define relative molecular mass.
The Relative Atomic Mass (Ar) of each atom in the molecule is added to form the Relative Molecular Mass (Mr).
The average mass of one molecule of a substance when compared to the mass of an atom of carbon or to one regular hydrogen atom is known as its relative molecular mass.
Although they differ from one another, molar mass and relative molar mass are connected. Avogadro's number of molecules is known as its molar mass. To determine the relative mass of a molecule, just combine the relative masses of its constituent elements.
2. Define the law of conservation of mass.
The law of mass conservation states that "Mass cannot be generated or destroyed in an isolated system, but it can be converted from one form to another.’ According to the law of conservation of mass, for a low-energy thermodynamic process, the mass of the reactants must equal the mass of the products.
Mass conservation is thought to be defined by a few assumptions from classical mechanics. With the help of quantum mechanics and special relativity, the law of conservation of mass was later changed to say that energy and mass are one conserved quantity.
























