




What is Solubility?
The highest amount of solute that may dissolve in a known quantity of solvent at a particular temperature is referred to as solubility.
A solution is a homogeneous mixture consisting of one or more solutes in a solvent. Salt in a cup of tea or coffee is a popular example of a solution. The property of solubility allows sugar molecules to dissolve in water. As a result, solubility can be defined as a material's (solute's) ability to dissolve in a certain solvent. According to various observations and experimental data, only polar solutes prefer to dissolve in polar solvents, whereas non-polar solvents dissolve only nonpolar solutes.
Solubility of Salts
Dissolution occurs when a solid solute is given to a solvent and the solute particles dissolve in the solvent. The process of crystallisation occurs when solute particles in a solution collide with one another and some of the particles separate from the solution.
A state of dynamic equilibrium is achieved between these two processes, when the number of solute molecules entering the solution matches the number of particles exiting the solution. As a result, the concentration of the solute in the solution will remain constant at any given temperature and pressure.
No additional solute can dissolve in the solvent after a given temperature and pressure, and a saturated solution is formed that has the maximum amount of solute. The concentration of a salt in a saturated solution at a certain temperature and pressure is referred to as salt solubility. A solution that is unsaturated allows for the addition of more solute.
Some soluble salts examples are Silver sulfite Ag2SO3, potassium chloride (KCl), sodium chloride (NaCl), magnesium chloride (MgCl2), and sodium sulphate Na2SO4. Sparingly soluble salts are of great significance to understand the concept of solubility as they reach equilibrium easily as compared to other salts hence the concentration can be found out. What is the importance of dissolved salts in water? Water dissolves many salts as it has a high dielectric constant, however solubility of salt in water depends on other factors also such as the nature of salt, temperature and pressure.
Factors Affecting Solubility of Salts
Effect of Temperature:
Temperature has a significant impact on solid solubility. According to Le Chatelier's Principle, if the dissolution process is endothermic, the solubility should increase as the temperature rises. Solid solubility decreases if the dissolution process is exothermic.
Effect of Pressure:
Solid solubility hardly gets affected by changes in pressure due to the fact that solids and liquids can’t be compressed and practically do not get affected by changes in pressure.
Polarity:
Solutes usually dissolve in solvents with similar polarities.
Solubility Product
The solubility product constant, abbreviated as Ksp, is a simplified equilibrium constant that describes the equilibrium between a solid and its corresponding ions in a solution. Its value indicates how easily a chemical dissociates in water. The more soluble a substance is, the higher is the solubility product constant. The product of concentration of the ion is the Ksp expression for a specific salt.
To obtain the solubility equilibrium, each concentration is raised to a power equal to the coefficient of that ion in a balanced equation. The solubility product constants are used to describe the saturated solutions of low-solubility ionic substances.
For example, silver chloride dissociates to less extent in the silver ions and chloride ions when added to water as it is sparingly soluble.
$\operatorname{AgCl}(\mathrm{s}) \leftrightarrow \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{Cl}^{-}(\mathrm{aq})$
The ksp is given by $\mathrm{Ksp}=\left[\mathrm{Ag}^{+}\right]\left[\mathrm{Cl}^{-}\right]$
Solubility Product Formula
The ionic compound and the undissolved solid in a saturated solution are said to be in a condition of dynamic equilibrium.
$M x A y(s) \rightarrow x M^{y+}(a q)+y A^{x-}(a q) \\ $ $K_{s p}=\left[M^{+y}\right]^{x}\left[A^{x-}\right]^{y}$
Common Ion Effect
The common ion effect is a result of Le Chatelier's principle for ionic association or dissociation equilibrium reactions.The solubility of a compound is also influenced by the common ion effect.When we add another species containing the same ion to an existing solution containing different types of ions and equilibrium is attained, we see a reduction in the degree of dissociation of the initial species. This phenomenon of reduction in degree of dissociation is known as the common ion effect.
For example,
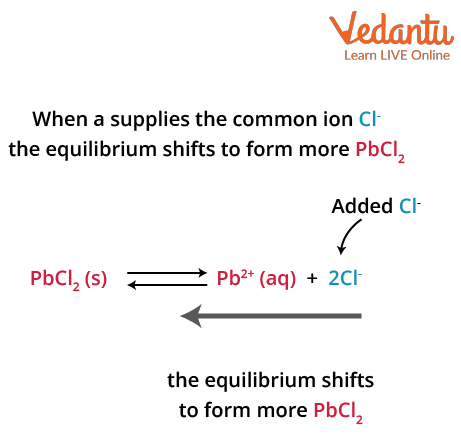
Common ion Effect in PbCl2
Conclusion
The ability of a substance known as a solute to dissolve in a solvent and create a solution is defined as solubility. Ionic chemicals that dissociate and create cations and anions in water have a wide range of solubility. Some substances are highly soluble, even absorbing moisture from the air, whereas others are highly insoluble. Ksp is a simplified equilibrium constant that represents the equilibrium between a solid and its equalient ions in a solution. Its value represents the ease with which a chemical dissociates in water. The higher the solubility product constant, the more soluble a material is.
FAQs on Solubility of Salts for JEE
1. What is the solubility curve?
The solubility curve depicts the change in solubility of a given material as a function of temperature. The solubility curve is a curved line displayed on a graph that depicts the relationship between temperature and a substance's solubility at different temperatures. The solubility curve is a graphical representation between solubility and temperature. The solubility curve shows how the solubility of a solid changes with temperature in a solvent. Temperature variations should be represented on the x-axis, while solubility should be plotted on the y-axis.
2. Do gases dissolve in water and can they be referred to as solutes?
On Earth, water is a good solvent. It is soluble in a wide range of substances. Everyone is familiar with solid-state compounds that can dissolve in water. Surprisingly, several chemicals that naturally exist in a gaseous state can dissolve in water. Carbon dioxide is a well-known gas for this purpose, and it's often used in the creation of soft beverages and soda water. Oxygen is soluble in water in small levels, yet it is critical for the living system that all water bodies support. Another gas that dissolves in water is ammonia.
























