
Common monomer present in Melamine Formaldehyde polymer and Bakelite is
(A) Formaldehyde
(B) Phenol
(C) Melamine
(D) Ethylene Glycol
Answer
232.8k+ views
Hint: It is an aldehydic simple chemical compound made of hydrogen, oxygen and carbon. It is a colourless compound which has a pungent smell.
Complete step by step solution:
Now, let us know about the formation of Melamine Formaldehyde
-Melamine (1, 3, 5-triamino-2, 4, 6-triazine) formaldehyde.
-Melamine formaldehyde (MF) polymers are primarily made up of melamine and formaldehyde with formaldehyde.
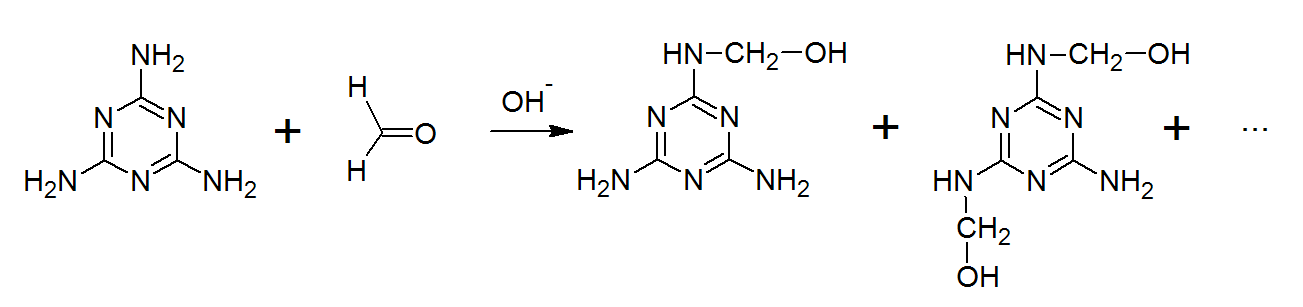
-Monomers of Melamine Formaldehyde are Melamine and Formaldehyde.
Additional Information:
-Melamine formaldehyde is a hard, durable, and versatile thermosetting amyloplast with good fire and heat resistance.
-It is an amino resin and has various material advantages, such as transparency, better hardness, thermal stability, excellent boil resistance, scratch resistance, abrasion resistance, flame retardant, moisture resistance and surface smoothness, which lead MF to large industrial applications.
Now, we learn the preparation of Bakelite
-Bakelite ${({C_6} - {H_6} - O.C - {H_2} - O)_x}$
-Bakelite is produced through condensation reaction between phenol and formaldehyde in the presence of either basic or acidic catalyst.
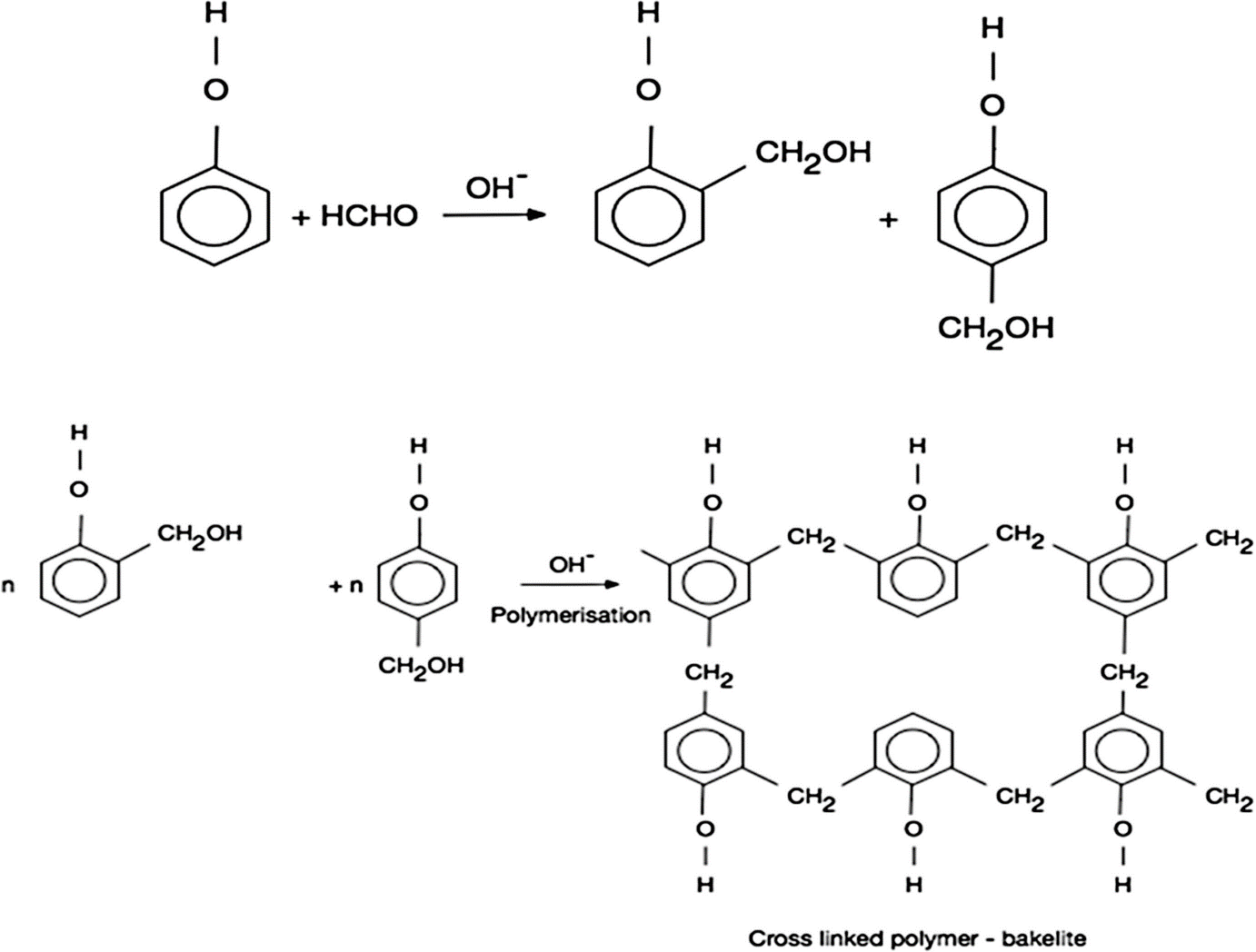
-Monomers of Bakelite are Phenol and Formaldehyde.
Hence, the common monomer of Melamine Formaldehyde and Bakelite is Formaldehyde. Therefore, Option (A)is correct.
Additional information:
-It is a thermo-setting and cross-linked polymer.
-Bakelite is a non-conductive and heat resistant material that makes it ideal for electrical insulators. It’s rigid, hard, scratch-resistant, infusible, water-resistant, insoluble solid and a good insulator.
-It was used for its non-conductive and heat-resistant properties in electrical insulators, and also in radios and telephones.
By learning the preparation of Bakelite and Melamine Formaldehyde we got to know that the monomers used for the preparation of Melamine are Melamine and Formaldehyde and the monomers used for the preparation of Bakelite are Formaldehyde and Phenol.
Note: 1. Phenol and Formaldehyde are required for the formation of Bakelite whereas Melamine and Formaldehyde are required for Melamine Formaldehyde polymer.
2. Do not get confused by the occurrence of Phenol and Melamine as the two are also monomers.
Complete step by step solution:
Now, let us know about the formation of Melamine Formaldehyde
-Melamine (1, 3, 5-triamino-2, 4, 6-triazine) formaldehyde.
-Melamine formaldehyde (MF) polymers are primarily made up of melamine and formaldehyde with formaldehyde.

-Monomers of Melamine Formaldehyde are Melamine and Formaldehyde.
Additional Information:
-Melamine formaldehyde is a hard, durable, and versatile thermosetting amyloplast with good fire and heat resistance.
-It is an amino resin and has various material advantages, such as transparency, better hardness, thermal stability, excellent boil resistance, scratch resistance, abrasion resistance, flame retardant, moisture resistance and surface smoothness, which lead MF to large industrial applications.
Now, we learn the preparation of Bakelite
-Bakelite ${({C_6} - {H_6} - O.C - {H_2} - O)_x}$
-Bakelite is produced through condensation reaction between phenol and formaldehyde in the presence of either basic or acidic catalyst.

-Monomers of Bakelite are Phenol and Formaldehyde.
Hence, the common monomer of Melamine Formaldehyde and Bakelite is Formaldehyde. Therefore, Option (A)is correct.
Additional information:
-It is a thermo-setting and cross-linked polymer.
-Bakelite is a non-conductive and heat resistant material that makes it ideal for electrical insulators. It’s rigid, hard, scratch-resistant, infusible, water-resistant, insoluble solid and a good insulator.
-It was used for its non-conductive and heat-resistant properties in electrical insulators, and also in radios and telephones.
By learning the preparation of Bakelite and Melamine Formaldehyde we got to know that the monomers used for the preparation of Melamine are Melamine and Formaldehyde and the monomers used for the preparation of Bakelite are Formaldehyde and Phenol.
Note: 1. Phenol and Formaldehyde are required for the formation of Bakelite whereas Melamine and Formaldehyde are required for Melamine Formaldehyde polymer.
2. Do not get confused by the occurrence of Phenol and Melamine as the two are also monomers.
Recently Updated Pages
JEE Main 2026 Session 2 Registration Open, Exam Dates, Syllabus & Eligibility

JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

Trending doubts
JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding Average and RMS Value in Electrical Circuits

Understanding Collisions: Types and Examples for Students

Ideal and Non-Ideal Solutions Explained for Class 12 Chemistry

Understanding Atomic Structure for Beginners

AssertionIn electrolytic refining of metal impure metal class 12 chemistry JEE_Main

Other Pages
JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions Hindi Medium (2025-26)

CBSE Class 12 Chemistry Set 1 56/2/1 2025: Question Paper, Answers & Analysis

CBSE Class 12 Chemistry Question Paper Set 3 2025 with Answers

Inductive Effect and Its Role in Acidic Strength

Degree of Dissociation: Meaning, Formula, Calculation & Uses




