
How many dichlorocyclohexane would be obtained on chlorination of chlorocyclohexane (including stereoisomers)?
(A) 4
(B) 6
(C) 8
(D) 9
Answer
233.1k+ views
Hint: The chlorination is the process of the addition of chlorine atoms. The chlorocyclohexane when treated with the chlorine molecule forms dichlorocyclohexane. The dichlorocyclohexane has two chlorine atoms attached to the cyclohexane ring. The positions of the chlorine atoms may vary concerning each other leading to the formation of different stereoisomers in the space.
Complete step by step solution:
Halogens ($\text{ C}{{\text{l}}_{\text{2}}}\text{ }$ and \[\text{ B}{{\text{r}}_{\text{2}}}\text{ }\]) reacts with the alkanes or the cycloalkanes in the presence of ultraviolet light to form haloalkanes. This reaction is called the free radical substitution reaction and gives a mixture of mono, di, or polysubstituted haloalkanes which are difficult to separate into the pure compound.
The addition of the chlorine atom to the alkanes for the preparation of the haloalkanes is known as the chlorination. The free radical chlorination is taking place in the ultraviolet light.
In presence of sunlight or ultraviolet radiation, the bond between the chlorine molecules $\text{ C}{{\text{l}}_{\text{2}}}\text{ }$ undergoes the homolytic fission. Such that the $\text{ C}{{\text{l}}_{\text{2}}}\text{ }$ molecules break down to form two chlorine radicals. the homolytic fission of $\text{ C}{{\text{l}}_{\text{2}}}\text{ }$ molecule is as shown below,
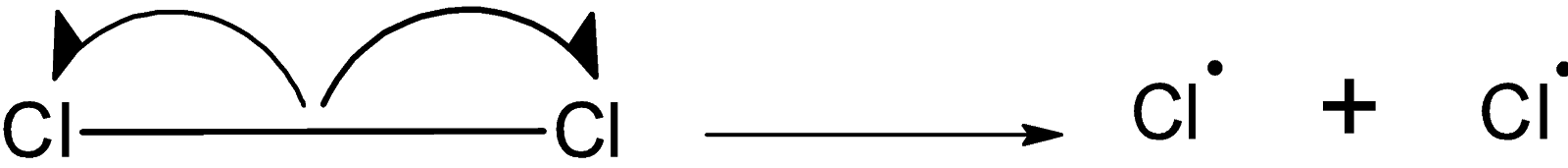
The chlorine radical attacks on the cyclohexane such that the hydrogen atom is replaced by the chlorine atom the reaction for the synthesis of cyclohexane is as shown below:
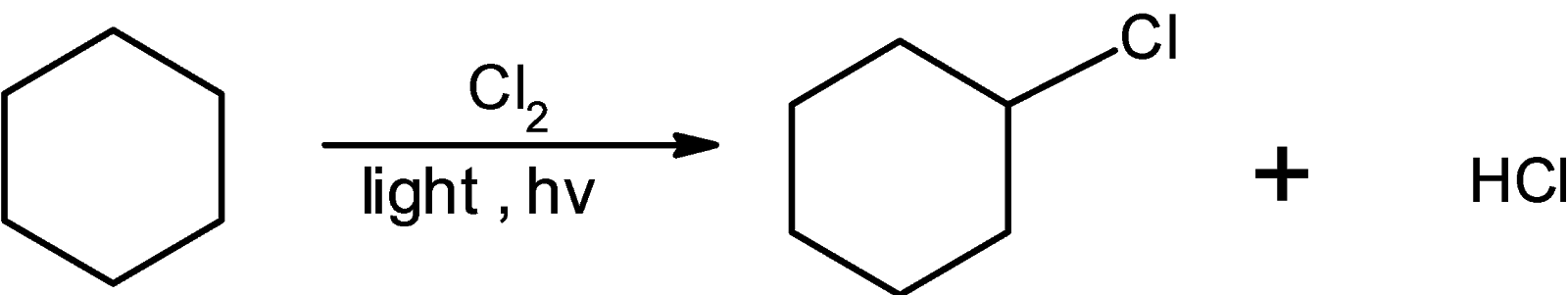
When mono substituted haloalkanes are further treated with the halogen results in the disubstituted haloalkanes. The chlorocyclohexane when treated with the excess of chlorine it is converted into the dichlorocyclohexane.
When chloro cyclohexane is treated with the chlorine, we get the ortho ( or 1,2 ), meta ( or 1,3 ), and the para ( or 1, 4) disubstituted product. The obtained dichlorocyclohexane products are as follows,

Let's have a look at the stereoisomers of each product obtained.
a) Ortho ( or 1,2 ) dichlorocyclohexane: the ortho-dichlorocyclohexane has the three stereoisomers. The one isomer in which the two bonds are above the plane and one when the two bonds $\text{ (C}-\text{H})\text{ }$ are below the plane. The ortho product has one more stereoisomer where the one bond is above and below the plane. The three stereoisomers of the ortho-dichlorocyclohexane are as follows:
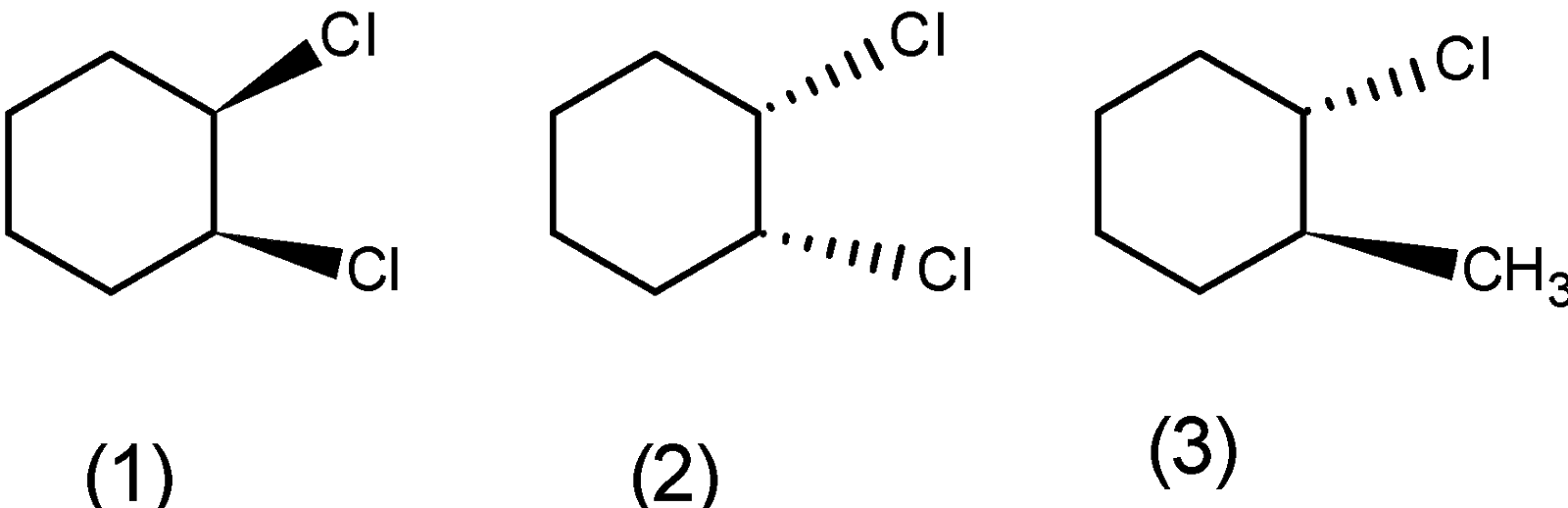
b) The meta-dichlorocyclohexane has three stereoisomers. One in which the two bonds are above the plane, below the plane, and the third stereoisomer in which the one bond is above and one is below the plane. The stereoisomers are as follows,
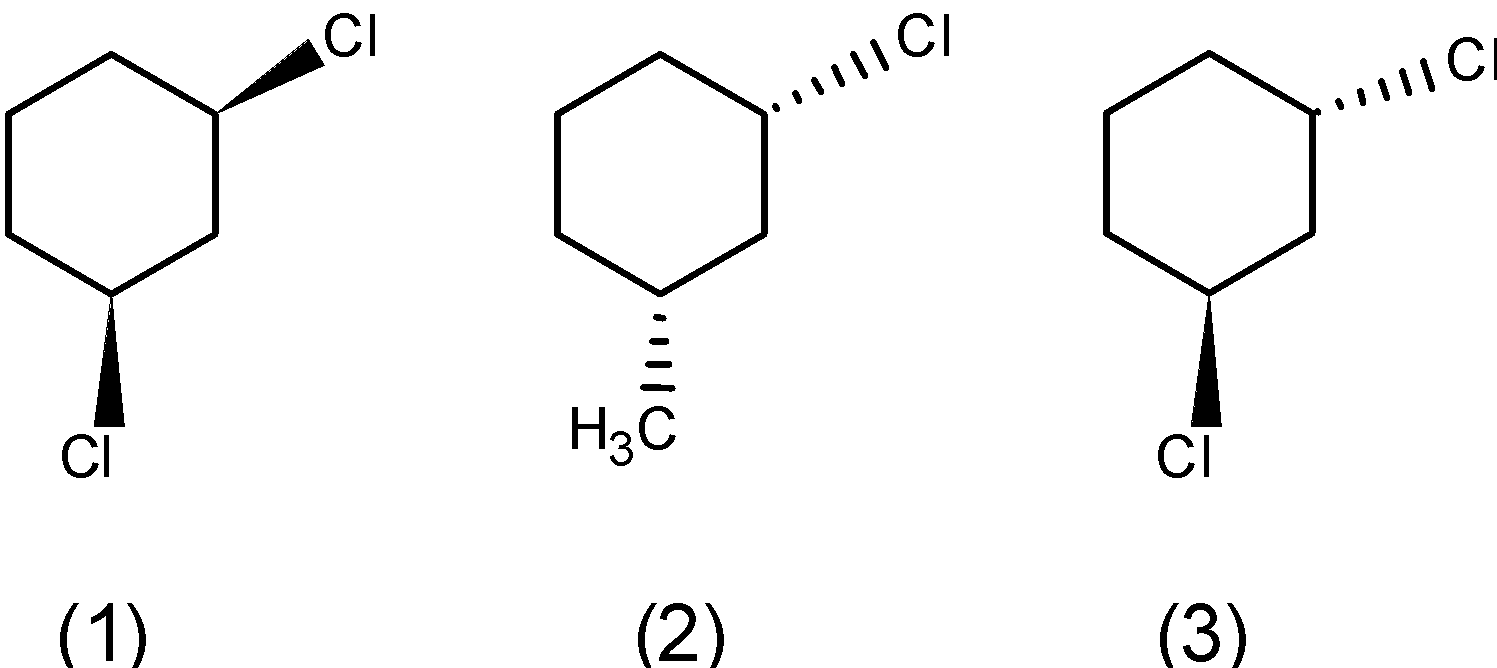
c) para-dichlorocyclohexane:
The para isomer of dichlorocyclohexane shares the plane of symmetry. The plane of symmetry passes through the two chlorine atoms. Thus, the para isomer can be expressed into stereoisomers. Thus, it has two isomers.
d) In these structures, the two chlorine atoms are linked to one carbon atom. As carbon is bonded to the two chlorine atoms hence it does not depict stereoisomerism. It has an achiral carbon centre. Therefore, only one structure is possible.
Therefore, the total number of structures possible for the chlorocyclohexane is equal to,
$\text{ Total number of structure = 3 + 3+ 2 + 2 = 9 }$
A total of 9 isomers is possible.
Hence, (D) is the correct option.
Note: The chlorine molecule gives a highly reactive radical. In excess of chlorine instead of one product, we may obtain the mixture of the product (di, tri, or polysubstituted chloro alkanes). Here, the second chlorine atom may take the 2, 3, or the 4 positions concerning the first position chlorine atoms. However, there is also a possibility of chlorine at the same carbon. This carbon is achiral as is linked to the same carbon atom and the molecules now possess the plane of symmetry.
However, the bromine radical is highly selective. Instead of forming a mixture of products, it has given one major product.
Complete step by step solution:
Halogens ($\text{ C}{{\text{l}}_{\text{2}}}\text{ }$ and \[\text{ B}{{\text{r}}_{\text{2}}}\text{ }\]) reacts with the alkanes or the cycloalkanes in the presence of ultraviolet light to form haloalkanes. This reaction is called the free radical substitution reaction and gives a mixture of mono, di, or polysubstituted haloalkanes which are difficult to separate into the pure compound.
The addition of the chlorine atom to the alkanes for the preparation of the haloalkanes is known as the chlorination. The free radical chlorination is taking place in the ultraviolet light.
In presence of sunlight or ultraviolet radiation, the bond between the chlorine molecules $\text{ C}{{\text{l}}_{\text{2}}}\text{ }$ undergoes the homolytic fission. Such that the $\text{ C}{{\text{l}}_{\text{2}}}\text{ }$ molecules break down to form two chlorine radicals. the homolytic fission of $\text{ C}{{\text{l}}_{\text{2}}}\text{ }$ molecule is as shown below,

The chlorine radical attacks on the cyclohexane such that the hydrogen atom is replaced by the chlorine atom the reaction for the synthesis of cyclohexane is as shown below:

When mono substituted haloalkanes are further treated with the halogen results in the disubstituted haloalkanes. The chlorocyclohexane when treated with the excess of chlorine it is converted into the dichlorocyclohexane.
When chloro cyclohexane is treated with the chlorine, we get the ortho ( or 1,2 ), meta ( or 1,3 ), and the para ( or 1, 4) disubstituted product. The obtained dichlorocyclohexane products are as follows,

Let's have a look at the stereoisomers of each product obtained.
a) Ortho ( or 1,2 ) dichlorocyclohexane: the ortho-dichlorocyclohexane has the three stereoisomers. The one isomer in which the two bonds are above the plane and one when the two bonds $\text{ (C}-\text{H})\text{ }$ are below the plane. The ortho product has one more stereoisomer where the one bond is above and below the plane. The three stereoisomers of the ortho-dichlorocyclohexane are as follows:

b) The meta-dichlorocyclohexane has three stereoisomers. One in which the two bonds are above the plane, below the plane, and the third stereoisomer in which the one bond is above and one is below the plane. The stereoisomers are as follows,

c) para-dichlorocyclohexane:
The para isomer of dichlorocyclohexane shares the plane of symmetry. The plane of symmetry passes through the two chlorine atoms. Thus, the para isomer can be expressed into stereoisomers. Thus, it has two isomers.
d) In these structures, the two chlorine atoms are linked to one carbon atom. As carbon is bonded to the two chlorine atoms hence it does not depict stereoisomerism. It has an achiral carbon centre. Therefore, only one structure is possible.
Therefore, the total number of structures possible for the chlorocyclohexane is equal to,
$\text{ Total number of structure = 3 + 3+ 2 + 2 = 9 }$
A total of 9 isomers is possible.
Hence, (D) is the correct option.
Note: The chlorine molecule gives a highly reactive radical. In excess of chlorine instead of one product, we may obtain the mixture of the product (di, tri, or polysubstituted chloro alkanes). Here, the second chlorine atom may take the 2, 3, or the 4 positions concerning the first position chlorine atoms. However, there is also a possibility of chlorine at the same carbon. This carbon is achiral as is linked to the same carbon atom and the molecules now possess the plane of symmetry.
However, the bromine radical is highly selective. Instead of forming a mixture of products, it has given one major product.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




