
On oxymercuration-demercuration, the given compound produces the major product:
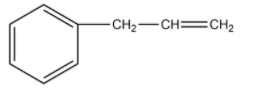
(A) 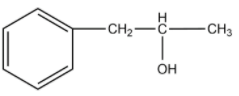
(B) 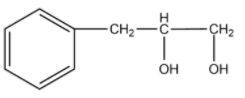
(C) 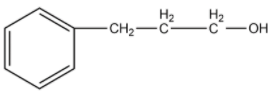
(D) 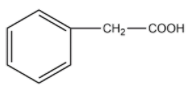
Answer
233.1k+ views
Hint: Recollect what happens in the oxymercuration-demercuration reaction. Identify which is the functional group of the reactant given in the question. Write the reaction equation and then determine which will be the most stable product to get the answer.
Complete step by step solution:
-Oxymercuration-demercuration is a two-step addition reaction which converts alkene to alcohol.
-In oxymercuration reaction, alkene reacts with mercuric acetate, ${{\left( C{{H}_{3}}COO \right)}_{2}}Hg$ in aqueous solution, which leads to addition of mercurous acetate$\left( C{{H}_{3}}COOHg \right)$ group and a hydroxyl group, -OH across the double bond.
-In the Demercuration reaction, the mercurous acetate$\left( C{{H}_{3}}COOHg \right)$ group will be replaced by hydrogen to form alkane.
-General reaction is represented as follows,
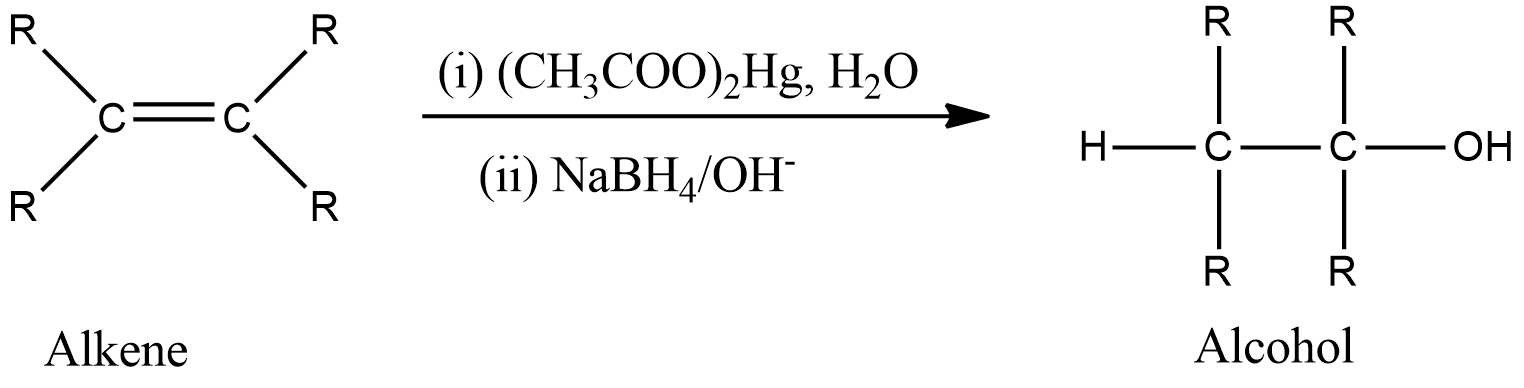
-The mechanism is shown below. First, there is the addition of mercuric acetate across the double bond to form a tricyclic intermediate which on hydrolysis forms an intermediate containing mercurous acetate group and alcohol group. Then, in the presence of reducing agents, demercuration occurs which removes mercurous acetate and reduces the intermediate to form the product that is alcohol.
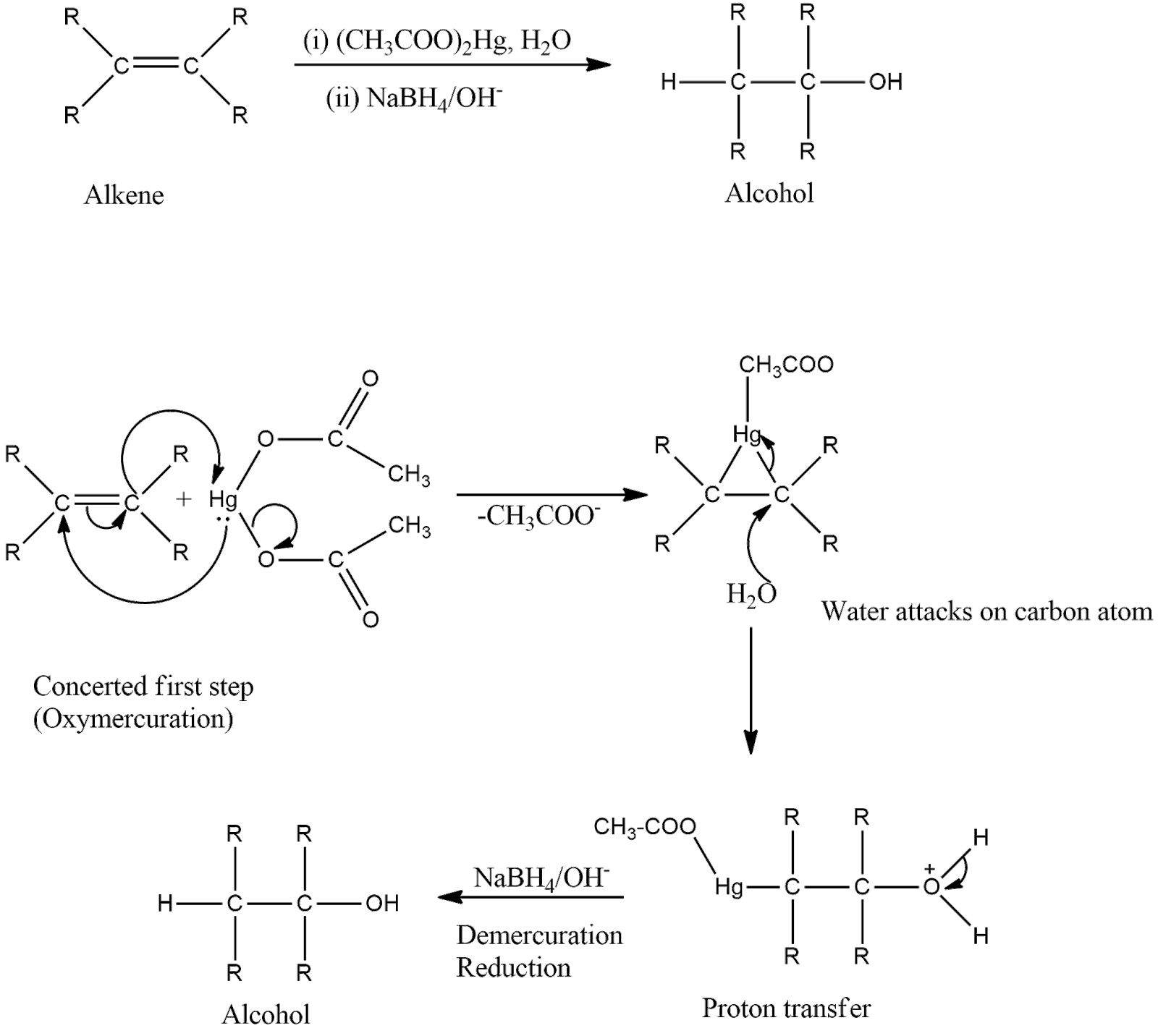
-In oxymercuration-demercuration reaction, carbocations are not formed, so rearrangements are not seen in this reaction.
-The reaction follows Markovnikoff's rule which states that the nucleophile will get added to that carbon atom in the double bond which has less number of hydrogen atoms and more number or substituents attached to it.
-The compound given as reactant is 3-phenylprop-1-ene. So, 3-phenylprop-1-ene will undergo oxymercuration-demercuration reaction to form 3-phenylpropan-2-ol and 3-phenylpropan-1-ol.
-But this reaction follows Markovnikoff’s rule and so, 3-phenylpropan-2-ol will be the major product.
-The reaction is represented as follows,
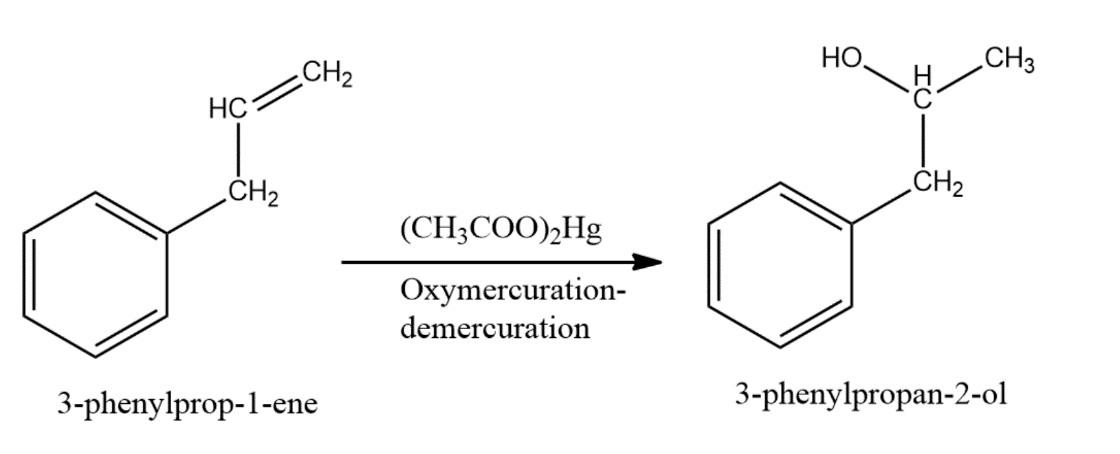
-Therefore, the given compound 3-phenylprop-1-ene on oxymercuration-demercuration reaction gives 3-phenylpropan-2-ol as the major product.
Therefore, the answer is option (A).
Note: In addition reactions of mixed unsaturated compounds, Markovnikoff’s rule is followed which tells us about the major product form in the reaction. Oxymercuration-demercuration reaction is an anti-addiction reaction and converts alkenes to neutral alcohols.
Complete step by step solution:
-Oxymercuration-demercuration is a two-step addition reaction which converts alkene to alcohol.
-In oxymercuration reaction, alkene reacts with mercuric acetate, ${{\left( C{{H}_{3}}COO \right)}_{2}}Hg$ in aqueous solution, which leads to addition of mercurous acetate$\left( C{{H}_{3}}COOHg \right)$ group and a hydroxyl group, -OH across the double bond.
-In the Demercuration reaction, the mercurous acetate$\left( C{{H}_{3}}COOHg \right)$ group will be replaced by hydrogen to form alkane.
-General reaction is represented as follows,
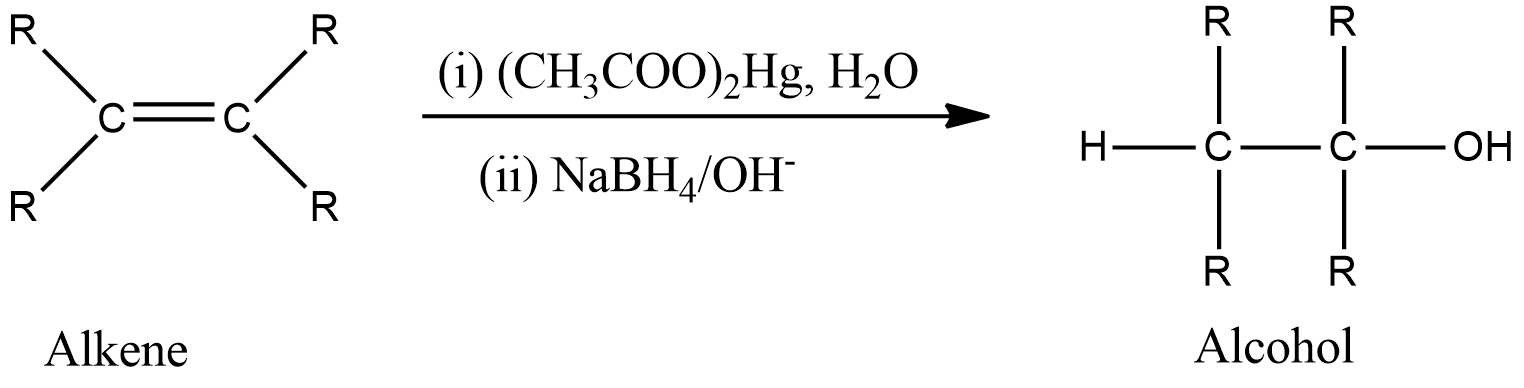
-The mechanism is shown below. First, there is the addition of mercuric acetate across the double bond to form a tricyclic intermediate which on hydrolysis forms an intermediate containing mercurous acetate group and alcohol group. Then, in the presence of reducing agents, demercuration occurs which removes mercurous acetate and reduces the intermediate to form the product that is alcohol.

-In oxymercuration-demercuration reaction, carbocations are not formed, so rearrangements are not seen in this reaction.
-The reaction follows Markovnikoff's rule which states that the nucleophile will get added to that carbon atom in the double bond which has less number of hydrogen atoms and more number or substituents attached to it.
-The compound given as reactant is 3-phenylprop-1-ene. So, 3-phenylprop-1-ene will undergo oxymercuration-demercuration reaction to form 3-phenylpropan-2-ol and 3-phenylpropan-1-ol.
-But this reaction follows Markovnikoff’s rule and so, 3-phenylpropan-2-ol will be the major product.
-The reaction is represented as follows,

-Therefore, the given compound 3-phenylprop-1-ene on oxymercuration-demercuration reaction gives 3-phenylpropan-2-ol as the major product.
Therefore, the answer is option (A).
Note: In addition reactions of mixed unsaturated compounds, Markovnikoff’s rule is followed which tells us about the major product form in the reaction. Oxymercuration-demercuration reaction is an anti-addiction reaction and converts alkenes to neutral alcohols.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




