




How Does Calorimetry Measure Heat Transfer?
Calorimetry is the branch of physics that deals with the quantitative measurement of heat exchanged during physical, chemical, or biological processes. It is fundamental in thermodynamics and is widely applied in scientific experiments to determine thermal properties such as specific heat, enthalpy, and heat capacity. Understanding calorimetry is crucial for analyzing the thermal interactions that govern various systems and reactions.
Definition and Significance of Calorimetry
Calorimetry refers to the process used to measure the amount of heat released or absorbed during a process. The main objective is to quantify heat transfer to or from substances as a result of chemical, physical, or biological changes. The term "calorimetry" originates from the Latin "calor," meaning heat, and is central in Introduction to Thermodynamics.
Principle of Calorimetry
The principle of calorimetry is based on the law of conservation of energy. When two or more bodies at different temperatures are brought into thermal contact, heat flows from the body at a higher temperature to the body at a lower temperature until thermal equilibrium is reached. The heat lost by the hotter body is numerically equal to the heat gained by the colder body if there is no loss to the surroundings.
This can be mathematically represented as:
$Q_{\text{lost}} = Q_{\text{gained}}$
The amount of heat exchanged can be further expressed as:
$Q = m \cdot s \cdot \Delta T$
where $m$ is mass, $s$ is specific heat capacity, and $\Delta T$ is the change in temperature.
Calorimeter: Construction and Working
A calorimeter is a device designed to measure the heat of chemical reactions or physical changes. It consists of a vessel made from a good conductor such as copper or aluminium, with insulation to prevent heat exchange with the environment. The calorimeter contains a thermometer and a stirrer to ensure uniform temperature throughout the contents.
The heat lost or gained by a sample placed inside the calorimeter is determined from the observed changes in temperature, considering the mass, specific heat capacity of the involved substances, and the calorimeter itself.
Types of Calorimeters
Different calorimetric devices are used depending on the nature of the process and the required precision. Each type operates on the principle of measuring heat exchange but follows different experimental approaches.
- Bomb calorimeter: used for combustion at constant volume
- Coffee-cup calorimeter: measures heat at constant pressure
- Adiabatic calorimeter: insulated to minimize heat loss
- Differential scanning calorimeter (DSC): measures differences in heat flow
- Isothermal titration calorimeter: measures heat of mixing or binding
The choice of calorimeter depends on the specific requirements of the experiment.
Calorimetry Equations and Heat Exchange Calculations
The general calorimetry equation relates heat exchange to mass, specific heat, and temperature change. For two substances mixed without heat loss to surroundings:
$m_1 s_1 (\theta_1 - \theta_f) = m_2 s_2 (\theta_f - \theta_2)$
Here, $\theta_1$ and $\theta_2$ are initial temperatures, $\theta_f$ is final equilibrium temperature, while $m$ and $s$ denote mass and specific heat capacities of the respective substances.
If the calorimeter has heat capacity $C$ and initial temperature $T_C$, its heat exchange is $C(\theta_f - T_C)$ and must be included in the heat balance equation.
Adiabatic and Non-Adiabatic Calorimetry
Adiabatic calorimeters are designed to prevent heat exchange with the environment, providing highly accurate measurements. In practice, perfect adiabatic conditions are challenging, and small correction factors may be applied for slight heat losses.
Non-adiabatic calorimetry allows some heat transfer to the surroundings, requiring additional corrections or averaging techniques to compensate for heat leaks during measurements.
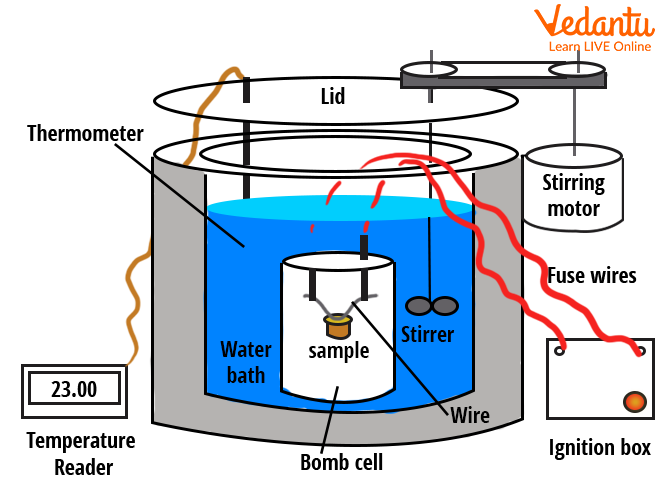
Bomb Calorimeter and Combustion Measurements
A bomb calorimeter is used to determine the heat of combustion of substances in a controlled, constant-volume environment. The sample is placed in a steel chamber (the bomb), filled with oxygen, then ignited. Heat released raises the temperature of water surrounding the bomb, which is recorded to calculate the heat of combustion.
| Component | Function |
|---|---|
| Bomb | Holds sample and oxygen |
| Water Jacket | Absorbs released heat |
| Stirrer | Ensures temperature uniformity |
| Thermometer | Monitors temperature rise |
Differential Scanning Calorimetry (DSC)
Differential Scanning Calorimetry (DSC) is a technique that measures the difference in heat flow into a sample and a reference as a function of temperature. It is primarily used for the thermal analysis of materials, including melting points, crystallization, glass transitions, and other thermal events.
The DSC technique aids in the study of enthalpy and entropy changes during phase transitions through temperature-dependent heat flow data.
DSC analysis is significant for polymer, pharmaceutical, and biological samples, where thermal stability and transitions are important.
Application Areas of Calorimetry
Calorimetry is widely used in various experimental and industrial fields to analyze heat effects and determine thermal characteristics. Some primary application areas include the following.
- Determining specific heat of solids and liquids
- Measuring enthalpy of reactions
- Assessing combustion energy of fuels
- Studying phase changes in materials
- Characterizing biochemical interactions
Calorimeter-based methods are crucial for reaction enthalpy measurements and metabolic energy estimation in biological systems.
Solved Example: Calorimetry Problem
A 100 g block of copper at $150^\circ$C is placed in 200 g of water at $25^\circ$C in a calorimeter of negligible heat capacity. Find the final temperature, assuming no heat loss to the surroundings. ($s_{\text{Cu}} = 0.39$ J/g·K, $s_{\text{water}} = 4.18$ J/g·K)
Heat lost by copper = heat gained by water:
$100 \times 0.39 \times (150 - T) = 200 \times 4.18 \times (T - 25)$
Solving gives $T \approx 27.3^\circ$C.
Key Points and Summary
- Calorimetry measures heat exchanged during physical or chemical changes
- Heat transfer follows the law of conservation of energy
- Types of calorimeters include bomb, adiabatic, and differential scanning calorimeters
- Used for specific heat, enthalpy, combustion, and phase study
- Accurate observation of temperature change is essential
A strong understanding of calorimetry is essential for solving problems related to heat exchange. Mastery of fundamental concepts assists in scoring well in competitive exams like JEE Main, where related topics such as Thermal Expansion Explained and Thermodynamics Revision Notes are also significant.
For a comprehensive overview and further resources on this topic, access the Calorimetry Overview.
FAQs on Understanding Calorimetry in Science
1. What is calorimetry in chemistry?
Calorimetry is the scientific process used to measure the amount of heat energy either absorbed or released during a chemical or physical change.
- It uses a device called a calorimeter.
- It helps in calculating enthalpy changes, heat capacity, and specific heat.
- Calorimetry is fundamental for studying thermodynamics and energy conversions in reactions.
- The measured heat change can help determine if a reaction is endothermic (absorbs heat) or exothermic (releases heat).
2. What is the principle of calorimetry?
The principle of calorimetry states that when two bodies at different temperatures are mixed, heat lost by the hot body is equal to the heat gained by the cold body, assuming no heat loss to the environment.
- This is based on the law of conservation of energy.
- Final temperature is reached when thermal equilibrium is established.
- The equation used is m₁c₁(θ₁ – θ) = m₂c₂(θ – θ₂), where m = mass, c = specific heat, θ = temperature.
- Accurate thermal insulation is crucial for reliable calorimetry results.
3. What is a calorimeter and how does it work?
A calorimeter is a device used to measure heat changes during a reaction or physical process.
- It has an insulated container to prevent heat loss.
- The system contains water and a thermometer.
- The substance or reaction is initiated inside, and the temperature change is recorded.
- Using the change in temperature, the amount of energy transferred can be calculated using Q = mcΔT.
4. What are the types of calorimeters?
There are several main types of calorimeters used in laboratories and industry:
- Simple calorimeter: For basic measurements of heat change in chemical reactions.
- Bomb calorimeter: Used for combustion reactions, especially to measure the calorific value of fuels.
- Coffee cup calorimeter: Used in school and undergraduate experiments for simple solution reactions.
- Differential scanning calorimeter (DSC): Used in advanced material and pharmaceutical research.
5. Define specific heat and explain its role in calorimetry.
Specific heat is the amount of heat energy required to raise the temperature of 1 gram of a substance by 1°C.
- In calorimetry, it helps determine how much energy is needed for temperature changes.
- The formula used is Q = mcΔT where c is the specific heat.
- Each substance has a unique specific heat value.
- Knowing specific heat helps in identifying unknown substances or comparing their heat capacities.
6. What is the difference between endothermic and exothermic reactions in calorimetry?
In calorimetry, endothermic reactions absorb heat and cause a temperature decrease in the surroundings, while exothermic reactions release heat and cause a temperature increase.
- Endothermic: Heat is taken in; product feels colder.
- Exothermic: Heat is given out; product feels warmer.
- Both are easily measured using a calorimeter, with the temperature change indicating the direction and amount of energy transfer.
7. What are the applications of calorimetry?
Calorimetry has wide applications in chemistry, physics, biology, and industry. Common uses include:
- Determining enthalpy changes in chemical reactions.
- Measuring calorific value of fuels and foods.
- Studying phase changes like melting, boiling, or freezing.
- Analyzing heat capacity of materials.
- Quality control in manufacturing (e.g., pharmaceuticals, polymers).
8. How is the heat capacity of a calorimeter determined?
To find the heat capacity of a calorimeter, a known quantity of hot and cold water is mixed and the resultant temperature change is measured.
- Heat lost by hot water = heat gained by cold water + calorimeter.
- Using the equation C_cal = (m_{hot}c_{water}(T_{hot} - T_{final}) - m_{cold}c_{water}(T_{final} - T_{cold}))/ (T_{final} - T_{cold}).
- Accurate measurements ensure the calorimeter's heat capacity is factored into further experiments.
9. How is calorimetry used to determine the heat of reaction?
Calorimetry determines the heat of reaction (enthalpy change) by measuring the change in temperature when reactants react in an insulated calorimeter.
- Reactants are mixed, and temperature is observed before and after the reaction.
- The heat absorbed or released is calculated using Q = mcΔT.
- For accurate values, corrections for calorimeter heat capacity and possible heat losses are made.
- This is essential for thermochemical calculations in chemistry exams.
10. What are the common sources of error in calorimetry experiments?
Common sources of error in calorimetry include heat loss to the environment, inaccurate measurement, and improper mixing.
- Heat exchange with surroundings (not perfectly insulated).- Incomplete reaction or mixing.
- Instrument calibration errors, such as faulty thermometer.- Evaporation of liquids during measurement.
- Not correcting for calorimeter heat capacity.
Minimizing these errors ensures reliable, exam-ready results.
11. Why is it important to use a well-insulated calorimeter?
A well-insulated calorimeter prevents heat exchange with the environment, ensuring accurate measurement of heat flow.
- Insulation reduces heat loss or gain from surroundings.
- Ensures that all measured temperature changes are due to the experiment only.
- Promotes reliable calculation of enthalpy change and specific heat values, which is important for exam scores and lab safety.

































