




Key Discoveries from Rutherford's Gold Foil Experiment
Rutherford's Alpha Scattering Experiment: Core Concept
Rutherford's alpha scattering experiment was a turning point in atomic physics, revealing that atoms have a small, dense, positively charged nucleus. This experiment used alpha particles to probe the structure of gold atoms and transformed our understanding of atomic models.
Before Rutherford, most scientists believed in the “plum pudding” model, which suggested atoms were blobs of positive charge with electrons embedded. Rutherford’s study overturned this, paving the way for the nuclear model of the atom.
The Experimental Setup and Process
In Rutherford's setup, a source of alpha particles directed a narrow beam at an extremely thin gold foil. Surrounding the foil, a zinc sulfide screen detected scattered alpha particles by creating scintillations when struck.
The experiment required the gold foil to be only a few atoms thick, ensuring most alpha particles interacted with single atoms. Positioning the detector all around allowed measurement of deflection angles with precision.
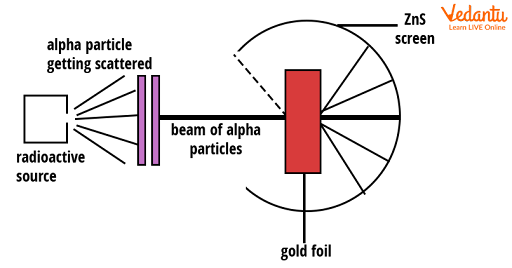
Alpha particles, being heavy and positively charged, were ideal for probing the internal structure of atoms. Their high energy meant they could penetrate the thin gold foil and potentially interact closely with atomic nuclei.
Key Observations and Unexpected Results
Most alpha particles passed straight through the gold foil without any significant deflection, indicating that atoms are mostly empty space. This observation was completely unexpected under previous atomic models.
A small fraction of particles were deflected at large angles, and an even smaller number rebounded nearly straight back. These rare, extreme deviations could only be explained by the presence of a concentrated positive mass in the center of the atom.
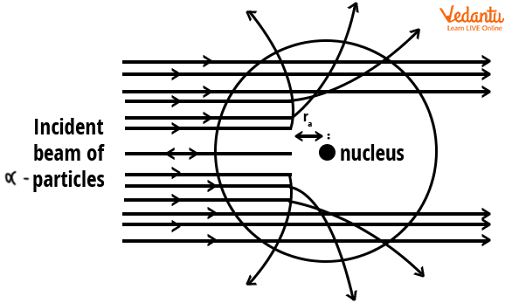
These results provided direct experimental evidence that the atom is not a uniform sphere, but has a tiny, dense nucleus with positive charge, surrounded mainly by empty space.
Rutherford’s Interpretation and the Nuclear Model
Rutherford concluded that almost the entire mass and positive charge of the atom is concentrated in a small nucleus at the center. The electrons, being much lighter, orbit this nucleus in the empty space around it.
This discovery explained why only very few alpha particles were deflected at large angles: they came extremely close to the dense nucleus, experiencing strong electrostatic repulsion. Most alpha particles missed the nucleus and continued straight.
The nuclear model replaced the plum pudding model and is the basis for modern atomic theory. This experiment also highlighted gaps in understanding, especially regarding atomic stability, which were later addressed by quantum models.
Rutherford Scattering Formula: Physics and Application
The formula that describes how many particles are scattered through a specific angle is called the Rutherford differential cross section. It quantifies the probability of an alpha particle being scattered by a given angle.
The mathematical expression for this differential cross-section is:
$ \left( \dfrac{d\sigma}{d\Omega} \right) = \left( \dfrac{1}{4\pi\epsilon_0} \right)^2 \left( \dfrac{Z_1Z_2e^2}{4E} \right)^2 \csc^4\left( \dfrac{\theta}{2} \right) $
Here, $ Z_1 $ and $ Z_2 $ are the atomic numbers of the alpha particle and target nucleus, $ e $ is the elementary charge, $ E $ is the kinetic energy, $ \epsilon_0 $ is the permittivity of free space, and $ \theta $ is the scattering angle.
This formula shows that as the scattering angle increases, the number of particles scattered at that angle rapidly decreases, matching experimental data and validating Rutherford’s model.
For further insights into atomic and nuclear phenomena, see Atom And Nuclei Overview, as theories built upon Rutherford’s findings.
Analogy: Solar System Model of the Atom
Rutherford’s atomic model is often compared to the solar system: the nucleus acts like the sun, and electrons revolve around it like planets. However, unlike planets, electrons are subject to different physical laws due to their charge and mass.
This analogy helps visualize atomic structure, but it also highlights the need for quantum mechanics. Classical orbits would make electrons lose energy and spiral into the nucleus—a limitation of Rutherford’s initial model.
Types of Alpha Particle Interactions
- Alpha particles passing far from the nucleus remain undeviated.
- Alpha particles passing near the nucleus experience moderate deflection.
- Alpha particles heading directly at the nucleus are deflected strongly.
This pattern occurs because the electrostatic repulsive force is strongest near the dense nucleus and negligible further away, supporting the idea that the nucleus contains most of the atom’s mass and charge.
Numerical Example: Calculating Rutherford Scattering
Suppose an alpha particle with kinetic energy $E = 6 \times 10^{-14}$ J scatters off a gold nucleus ($Z = 79$) at an angle $\theta = 60^\circ$. Find the differential cross-section.
Given: $Z_1 = 2$, $Z_2 = 79$, $e = 1.6 \times 10^{-19}$ C, $E = 6 \times 10^{-14}$ J, $ \epsilon_0 = 8.85 \times 10^{-12} $ F/m, $\theta = 60^\circ$.
Formula: $ \left( \dfrac{d\sigma}{d\Omega} \right) = \left( \dfrac{1}{4\pi\epsilon_0} \right)^2 \left( \dfrac{Z_1 Z_2 e^2}{4E} \right)^2 \csc^4 \left( \dfrac{\theta}{2} \right) $
Substitute the values: $ \left( \dfrac{d\sigma}{d\Omega} \right) = \left( \dfrac{1}{4\pi \times 8.85 \times 10^{-12}} \right)^2 \left( \dfrac{2 \times 79 \times (1.6 \times 10^{-19})^2}{4 \times 6 \times 10^{-14}} \right)^2 \csc^4 30^\circ $
Calculate $ \csc 30^\circ = 2 $, so $ \csc^4 30^\circ = 16 $.
Now, compute stepwise: The constants and substitutions yield $ \dfrac{d\sigma}{d\Omega} \approx 1.7 \times 10^{-26} $ m$^2$/steradian.
Therefore, the probability of scattering at $60^\circ$ is very low, confirming that few particles are deflected at large angles. This matches experimental findings.
Comparison Table: Thomson vs. Rutherford Models
| Thomson Model | Rutherford Model |
|---|---|
| No nucleus; positive charge spread | Central dense nucleus present |
| Electrons mixed in "pudding" | Electrons orbit nucleus |
| Fails to explain alpha scattering | Explains scattering patterns |
| No empty space in atom | Mostly empty space |
Significance for Modern Physics and JEE Exams
Mastering Rutherford’s alpha scattering experiment is crucial for exams such as JEE. It directly supports understanding concepts like the structure of the nucleus, properties of particles, and experimental validation in physics.
The experiment also forms the foundation for advanced topics, including nuclear fission and fusion processes. For more on these, explore Nuclear Fission And Fusion Explained for extended learning.
Applications and Real-World Impact
- Determining nuclear size and atomic structure
- Pioneering nuclear physics research methods
- Radioactive tracing and medical imaging advances
- Development of nuclear energy technologies
Rutherford’s findings influenced countless breakthroughs in nuclear physics. For further context on related radioactive processes, see Alpha, Beta, And Gamma Decay.
Limitations and Evolution of the Model
Rutherford's model could not explain atomic stability or discrete spectral lines. Electrons, according to classical physics, would spiral into the nucleus, but this does not happen in reality.
Further refinements led to quantum atomic models, offering explanations for atomic energy levels and stability. For insight into nuclear binding, read Understanding Binding Energy.
Practice Question: JEE-Level Challenge
A $5$ MeV alpha particle is fired at a thin gold foil. Calculate the scattering angle if the impact parameter equals the gold nucleus radius. Use $Z = 79$.
Common Mistakes in Rutherford Scattering Experiment
- Assuming all deflections are due to electron clouds
- Neglecting statistical distribution of scattering angles
- Forgetting the atom’s large empty spaces
- Overlooking limitations on atomic stability
Care must be taken to apply the Rutherford scattering formula only in classical scenarios, as quantum effects become important with lighter nuclei or higher energies. In-depth experiment understanding is vital for success in competitive exams.
Rutherford’s method also inspired technologies like nuclear reactors. To explore how controlled nuclear reactions apply in modern energy, visit Nuclear Reactor Functionality.
Related Physics Topics
- Deepen concepts with Atom And Nuclei Overview
- Study nuclear reactions at Nuclear Fission And Fusion Explained
- Explore quantum effects via Photoelectric Effect Insights
- Understand radioactive decay at Alpha, Beta, And Gamma Decay
- Learn stability principles in Understanding Binding Energy
- Discover practical energy use with Nuclear Reactor Functionality
FAQs on Understanding Rutherford's Alpha Scattering Experiment
1. What is Rutherford's alpha scattering experiment?
Rutherford's alpha scattering experiment was a key experiment in atomic physics that revealed the existence of a small, dense atomic nucleus. In this experiment, alpha particles were directed at a thin gold foil, and their deflections were observed. The results showed that:
- Most alpha particles passed straight through the foil, indicating that most of the atom is empty space.
- Some particles were deflected at large angles, suggesting the presence of a dense, positively charged core called the nucleus.
- A few alpha particles bounced directly back, confirming the nucleus is very small and massive.
2. What conclusions did Rutherford draw from his gold foil experiment?
Rutherford concluded that the atom contains a small, dense, positively charged core, now known as the nucleus. The key conclusions were:
- The atom is mostly empty space.
- The nucleus contains most of the atom's mass and all its positive charge.
- Electrons move around the nucleus in the remaining space.
3. What are the main features of Rutherford's atomic model?
Rutherford's atomic model described the structure of atoms after analyzing the gold foil experiment. The main features are:
- Atoms have a small, dense nucleus at the center.
- The nucleus is positively charged and contains most of the atom's mass.
- Electrons revolve in orbits around the nucleus.
- Most of the atom is empty space.
4. Why did most alpha particles pass straight through the gold foil in Rutherford's experiment?
Most alpha particles passed through the gold foil because atoms are mainly empty space. As a result:
- Only a few particles encountered the small, dense nucleus.
- The majority experienced no deflection and continued their original paths.
5. What were the limitations of Rutherford's atomic model?
Rutherford's atomic model had several limitations, despite its groundbreaking discoveries:
- It could not explain the stability of electrons in orbits, as classical physics predicted they would spiral into the nucleus.
- It failed to explain atomic spectra and electron energy levels.
- Did not justify chemical properties and bonding structures.
6. Who performed the gold foil experiment and when?
Ernest Rutherford, with the help of Hans Geiger and Ernest Marsden, performed the gold foil experiment in 1909. This experiment was conducted at the University of Manchester and revolutionized the understanding of atomic structure.
7. What observations were made during Rutherford's alpha scattering experiment?
Three key observations were made in Rutherford's alpha scattering experiment:
- Most alpha particles passed straight through the gold foil without deflection.
- Some were deflected at small angles.
- A tiny fraction rebounded at angles greater than 90°, even coming backward.
8. How did Rutherford’s experiment challenge the plum pudding model of the atom?
Rutherford’s experiment proved that the atom is not a uniform mass, as proposed by the plum pudding model. Main points include:
- Most of the mass and positive charge is concentrated in a small nucleus.
- Electrons surround this nucleus in mostly empty space.
- The uniform distribution of charges in the atom was disproved.
9. Describe the setup of Rutherford's gold foil experiment.
The Rutherford gold foil experiment consisted of:
- A source emitting alpha particles (He2+ ions).
- A very thin sheet of gold foil.
- A zinc sulfide screen to detect scattered particles.
- Alpha particles were shot at the foil, and their scattering angles were recorded.
10. Why is Rutherford’s experiment considered significant in the history of atomic models?
Rutherford’s experiment is highly significant because it revealed the true structure of the atom. Its importance includes:
- Discovery of the atomic nucleus.
- Disproving earlier atomic models.
- Providing a foundation for modern atomic theory and quantum mechanics.
























