
The total number of optically active compounds formed in the following reaction is?
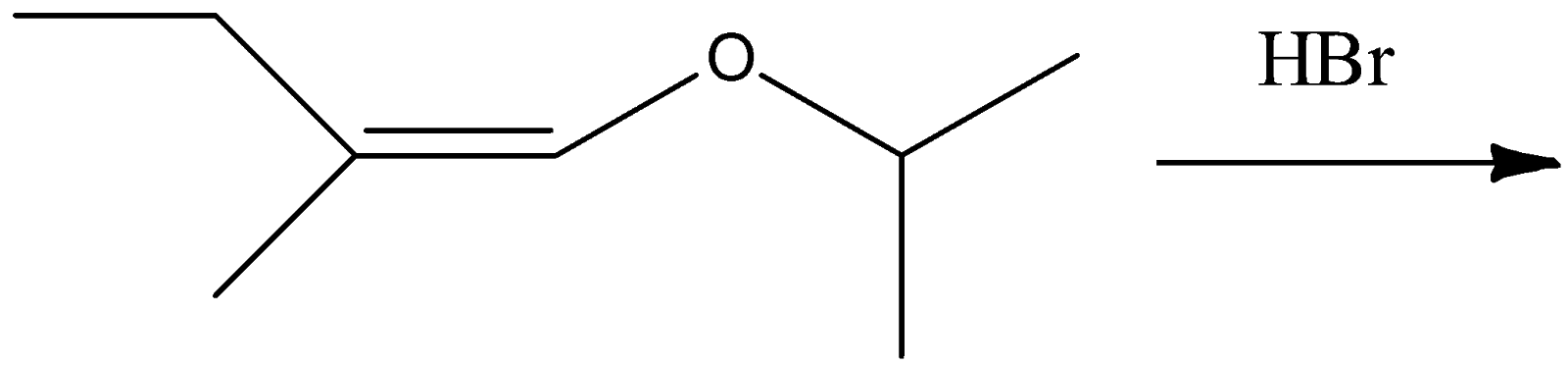
(A) Zero
(B) Six
(C) Four
(D) Two
Answer
232.8k+ views
Hint: This is an additional reaction to the alkene functional groups. A compound that has a chiral carbon can show optical isomerism. If an atom which can donate its lone pairs is bonded to the vinylic carbon, then it can give resonance structures.
Complete step by step solution:
Let’s see what will be the product of this reaction with an explanation.
It is an additional reaction where the addition of Hydrobromic acid is done at two carbons having a double bond. However, there is an oxygen atom which is directly bonded to the vinylic carbon. It will give resonance structures and will direct the position of the incoming electrophile. Let’s see how the addition will occur.
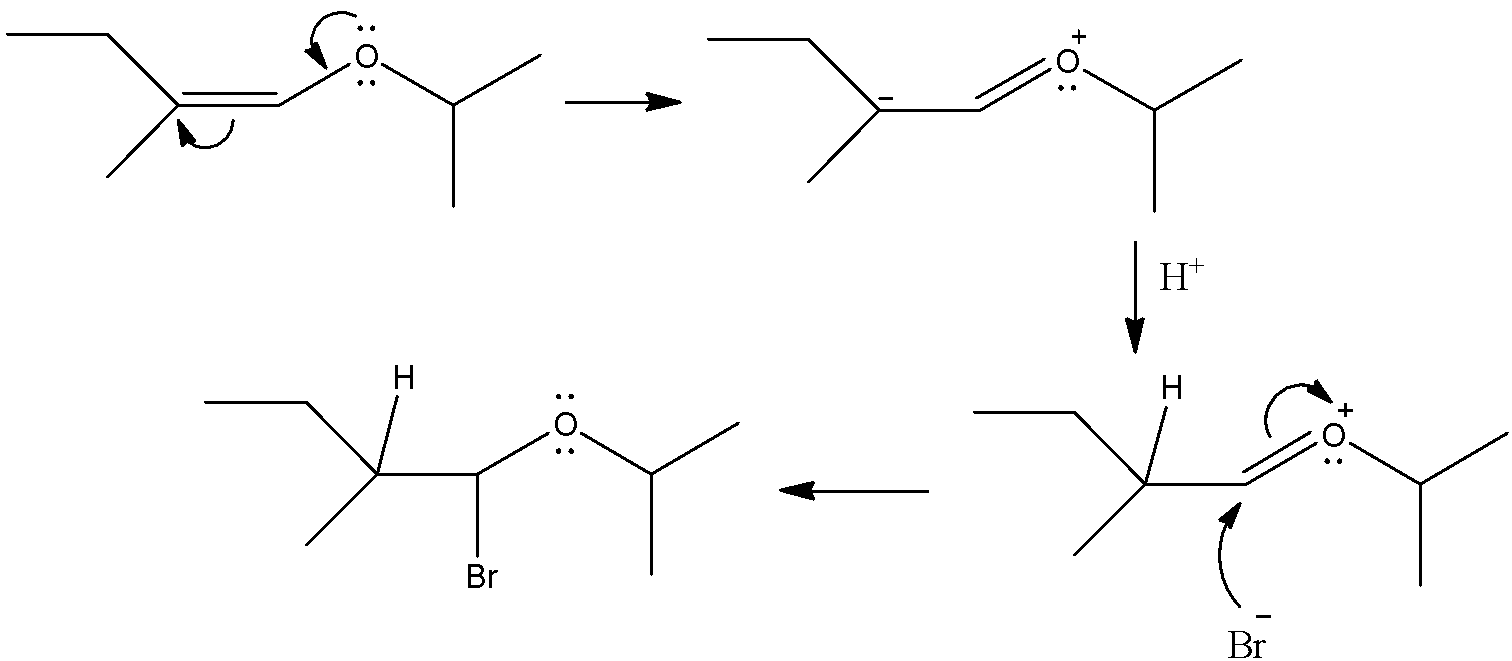
So, we can see that first oxygen will give its lone pair to the vinylic carbon and there will be a carbanion formed after the breakage of C-C double bond. This will attack on the proton and make a sigma bond with it. Then, the bromine atom which is an anion will attack the vinylic carbon and will make a sigma bond with it. The double bond between the carbon and oxygen will be broken and the positive charge on the oxygen atom will be neutralized.
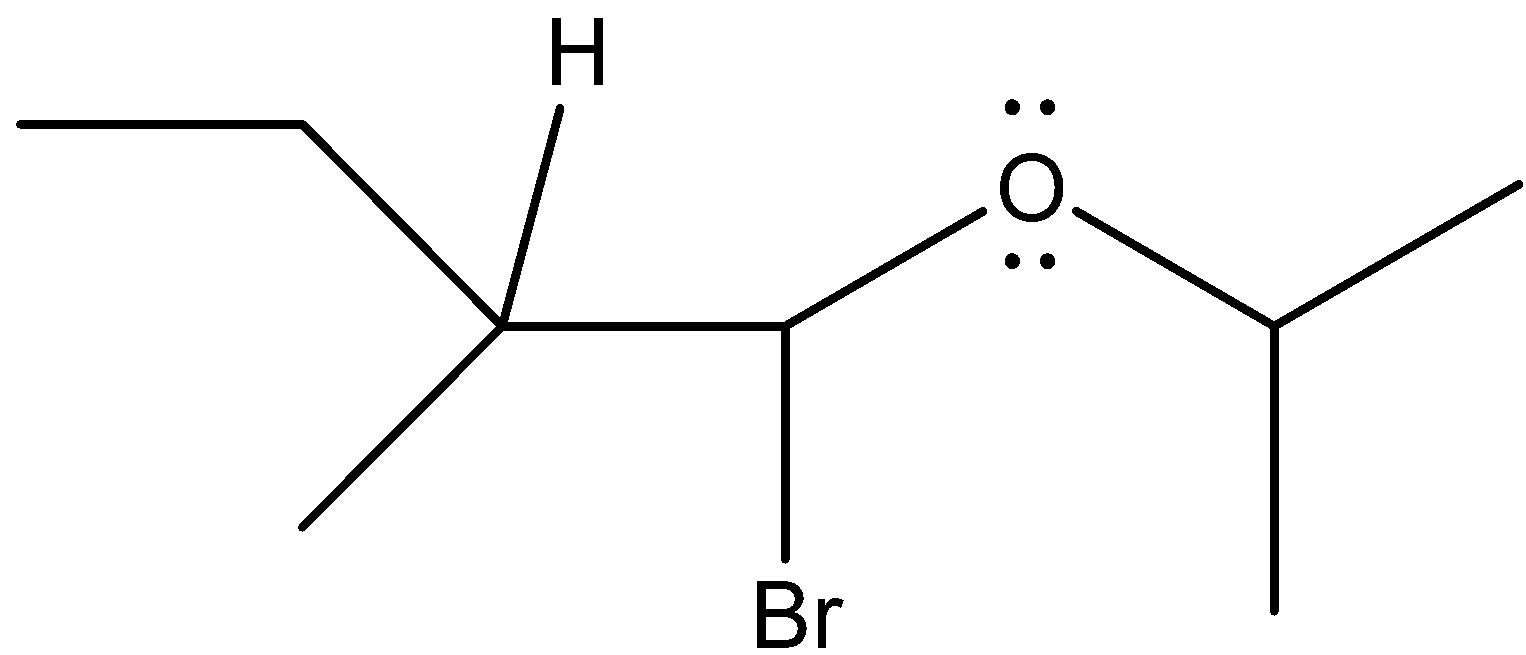
So, our final product can be written as
Now, see all the carbon atoms’ configuration.
- The carbon atom that has four different substituents connected with it by four single bonds, then the carbon is said to be chiral carbon.
In our product, carbon-bearing bromine atoms and the carbon bonded to that carbon is chiral because they have four different groups attached to fulfil their valency of 4 electrons.
-Any compound that has a chiral carbon, has optical isomers.
So, the number of optical isomers produced during this reaction as products=\[{{n}^{2}}\]
Here, n= number of chiral atoms
=\[{{(2)}^{2}}\]
=4
So, we can say that 4 optical isomers will be produced during this reaction. Their structures are as shown below.
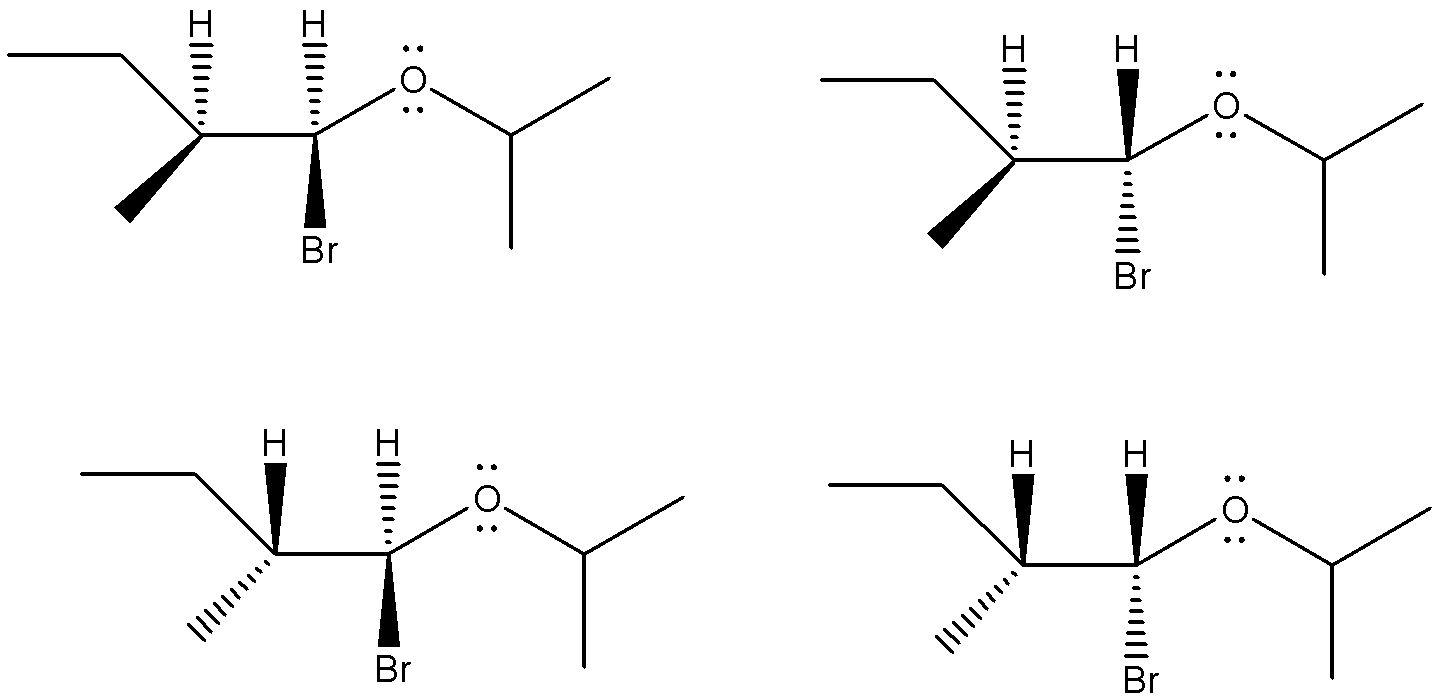
So, the correct answer is (C).
Note: Note that as oxygen is there which is directly bonded to the vinylic carbon. This will result in a product such that an anti-Markovnikov type of addition has occurred to the double bond. So, here anti-Markovnikov product is obtained even though peroxide is not present and that is because of the structure of the reactant.
Complete step by step solution:
Let’s see what will be the product of this reaction with an explanation.
It is an additional reaction where the addition of Hydrobromic acid is done at two carbons having a double bond. However, there is an oxygen atom which is directly bonded to the vinylic carbon. It will give resonance structures and will direct the position of the incoming electrophile. Let’s see how the addition will occur.

So, we can see that first oxygen will give its lone pair to the vinylic carbon and there will be a carbanion formed after the breakage of C-C double bond. This will attack on the proton and make a sigma bond with it. Then, the bromine atom which is an anion will attack the vinylic carbon and will make a sigma bond with it. The double bond between the carbon and oxygen will be broken and the positive charge on the oxygen atom will be neutralized.

So, our final product can be written as
Now, see all the carbon atoms’ configuration.
- The carbon atom that has four different substituents connected with it by four single bonds, then the carbon is said to be chiral carbon.
In our product, carbon-bearing bromine atoms and the carbon bonded to that carbon is chiral because they have four different groups attached to fulfil their valency of 4 electrons.
-Any compound that has a chiral carbon, has optical isomers.
So, the number of optical isomers produced during this reaction as products=\[{{n}^{2}}\]
Here, n= number of chiral atoms
=\[{{(2)}^{2}}\]
=4
So, we can say that 4 optical isomers will be produced during this reaction. Their structures are as shown below.

So, the correct answer is (C).
Note: Note that as oxygen is there which is directly bonded to the vinylic carbon. This will result in a product such that an anti-Markovnikov type of addition has occurred to the double bond. So, here anti-Markovnikov product is obtained even though peroxide is not present and that is because of the structure of the reactant.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




