
Which of the following radiations has the highest penetrating power?
A) $\alpha - rays$
B) $\beta - rays$
C) $\gamma - rays$
D) $X - rays$
Answer
232.8k+ views
Hint: The "rays" emitted by radioactive elements were named in order of their power to penetrate various materials, using the first three letters of the Greek alphabet: alpha rays as the least penetrating, followed by beta rays, followed by gamma rays as the most penetrating.
Complete step by step solution:
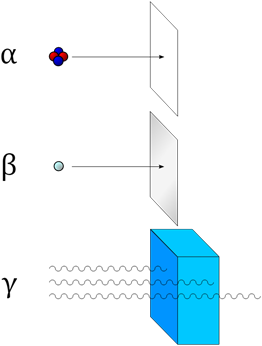
There are typically four types of radioactive radiation namely alpha radiation, beta radiation, gamma radiation, and x radiation. Neutron radiation is also encountered in nuclear power plants and high-altitude flight and emitted from some industrial radioactive sources.
Penetrating power refers to the energy with which the radiation particles are ejected from the atom. The higher the energy, the more the particles or light produced by radioactive decay will penetrate a substance.
Penetration of the modes of decay Alpha particles can be completely stopped by a sheet of paper. Beta particles can be stopped by aluminum shielding. Gamma rays can only be reduced by much more substantial mass, such as a very thick layer of lead.
X-rays are highly penetrating and interact with matter through ionization via three processes, photoelectric effect, Compton scattering or pair production. Their high penetration power the impact of X-rays can occur throughout a body, they are however less ionizing than alpha particles.
Hence the correct order of penetrating power of the given radiation is:
$\gamma > \beta > \alpha > X - rays$
Therefore the correct option of the given question is option (C).
Note: Gamma ray is the ray with the smallest wavelength but consists of the highest energy than any wave in the electromagnetic spectrum. Gamma rays are capable of emitting tons of energy in a split second than our sun in its entire billion year lifetime.
Complete step by step solution:
There are typically four types of radioactive radiation namely alpha radiation, beta radiation, gamma radiation, and x radiation. Neutron radiation is also encountered in nuclear power plants and high-altitude flight and emitted from some industrial radioactive sources.
Penetrating power refers to the energy with which the radiation particles are ejected from the atom. The higher the energy, the more the particles or light produced by radioactive decay will penetrate a substance.
Penetration of the modes of decay Alpha particles can be completely stopped by a sheet of paper. Beta particles can be stopped by aluminum shielding. Gamma rays can only be reduced by much more substantial mass, such as a very thick layer of lead.

X-rays are highly penetrating and interact with matter through ionization via three processes, photoelectric effect, Compton scattering or pair production. Their high penetration power the impact of X-rays can occur throughout a body, they are however less ionizing than alpha particles.
Hence the correct order of penetrating power of the given radiation is:
$\gamma > \beta > \alpha > X - rays$
Therefore the correct option of the given question is option (C).
Note: Gamma ray is the ray with the smallest wavelength but consists of the highest energy than any wave in the electromagnetic spectrum. Gamma rays are capable of emitting tons of energy in a split second than our sun in its entire billion year lifetime.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Dual Nature of Radiation and Matter Class 12 Physics Chapter 11 CBSE Notes - 2025-26

Understanding Uniform Acceleration in Physics

Understanding the Electric Field of a Uniformly Charged Ring

JEE Advanced Weightage 2025 Chapter-Wise for Physics, Maths and Chemistry

Derivation of Equation of Trajectory Explained for Students




