
Which one of the following is used in the preparation of cellulose nitrate?
(A) \[{\text{KN}}{{\text{O}}_{\text{3}}}\]
(B) \[{\text{HN}}{{\text{O}}_{\text{3}}}\]
(C) \[{\text{KN}}{{\text{O}}_2}\]
(D) \[{\text{HN}}{{\text{O}}_2}\]
Answer
233.1k+ views
Hint: Cellulose nitrate, also known as nitrocellulose is a very highly flammable compound. It is the resultant product obtained when a cellulose molecule is modified chemically through nitration reaction. Its first major application was in the preparation of guncotton as a replacement of gunpowder.
Complete step by step answer: Cellulose nitrate is generally prepared by the nitration of cellulose with nitric acid or with a mixture of nitric acid and some other acid, which is usually sulphuric acid or hydrochloric acid or with a very powerful nitrating agent.
Usually the production of nitrocellulose is done using the mixture of nitric acid and sulphuric acid, where sulphuric acid acts as the reaction catalyst. The repeating unit cellulose chain is a glucose unit containing three hydroxyl groups. Each of these three hydroxyl groups can form a nitrate ester. Therefore, it can be said that cellulose nitrate or nitrocellulose is a mononitrocellulose or dinitrocellulose or trinitrocellulose or a mixture of the three. Thus, nitrocellulose is not a nitro compound but a nitrate ester.
The chemical equation showing the reaction between cellulose and nitric acid for the preparation of cellulose nitrate is shown below.
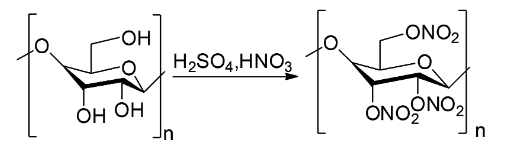
This reaction process gives about 85 percent yield of cellulose nitrate and the rest of the cellulose is converted to oxalic acid.
Out of the given options, only B has the option of \[{\text{HN}}{{\text{O}}_{\text{3}}}\] which is the chemical formula of nitric acid.
Hence, B is correct.
Note: The number of hydroxyl groups in nitrocellulose is less than that of parent cellulose molecules and so nitrocellulose molecules do not associate through hydrogen bonding. The main application of nitrocellulose is in the preparation of celluloid which is obtained by mixing nitrocellulose with camphor. Other major uses are the production of explosives and lacquers.
Complete step by step answer: Cellulose nitrate is generally prepared by the nitration of cellulose with nitric acid or with a mixture of nitric acid and some other acid, which is usually sulphuric acid or hydrochloric acid or with a very powerful nitrating agent.
Usually the production of nitrocellulose is done using the mixture of nitric acid and sulphuric acid, where sulphuric acid acts as the reaction catalyst. The repeating unit cellulose chain is a glucose unit containing three hydroxyl groups. Each of these three hydroxyl groups can form a nitrate ester. Therefore, it can be said that cellulose nitrate or nitrocellulose is a mononitrocellulose or dinitrocellulose or trinitrocellulose or a mixture of the three. Thus, nitrocellulose is not a nitro compound but a nitrate ester.
The chemical equation showing the reaction between cellulose and nitric acid for the preparation of cellulose nitrate is shown below.

This reaction process gives about 85 percent yield of cellulose nitrate and the rest of the cellulose is converted to oxalic acid.
Out of the given options, only B has the option of \[{\text{HN}}{{\text{O}}_{\text{3}}}\] which is the chemical formula of nitric acid.
Hence, B is correct.
Note: The number of hydroxyl groups in nitrocellulose is less than that of parent cellulose molecules and so nitrocellulose molecules do not associate through hydrogen bonding. The main application of nitrocellulose is in the preparation of celluloid which is obtained by mixing nitrocellulose with camphor. Other major uses are the production of explosives and lacquers.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




