




Haloform Reaction - Basic Concept
As we already know, aldehydes and ketones both possess the carbonyl functional group. They both undergo almost similar chemical reactions which include nucleophilic addition reactions, reduction, oxidation etc. In oxidation, haloform reactions are those reactions in which a halogen such as chlorine, bromine, or iodine, in the presence of hydroxide ions, reacts with ketones or aldehydes to produce carboxylate ions and haloforms. These reactions are mostly used to produce haloforms such as chloroform, bromoform or iodoform, and carboxylic acid. Compounds having at least one methyl group linked to the carbonyl carbon atom can undergo haloform reactions.
In this article, we are going to learn about the haloform reactions of ketones with reaction mechanisms and of acetaldehyde, which is the only aldehyde that undergoes haloform reaction.
What is a Haloform Reaction?
A haloform reaction is a type of oxidation reaction. In haloform reactions, haloforms such as chloroform $(CHC{{l}_{3}})$, bromoform $(CHB{{r}_{3}})$ or iodoform $(CH{{I}_{3}})$, and carboxylic acid are produced when aldehydes or ketones are reacted with halogens such as chlorine, bromine, or iodine in the presence of hydroxide ions. These reactions are mainly used to produce haloforms and to convert acetyl groups to carboxyl groups. Compounds having at least one methyl group linked to the carbonyl carbon atom can undergo haloform reactions. Aldehydes do not undergo haloform reactions except acetaldehyde.
Haloform Reaction
The reaction in which haloforms, i.e., halogenated carbon compounds such as chloroform, bromoform, iodoform, etc., are formed is termed as haloform reaction. It can be considered as the formation reaction of haloform.
Haloform Reaction Examples
The most common and typical examples of haloform reactions are listed below.
The conversion of acetophenone into sodium benzoate and iodoform
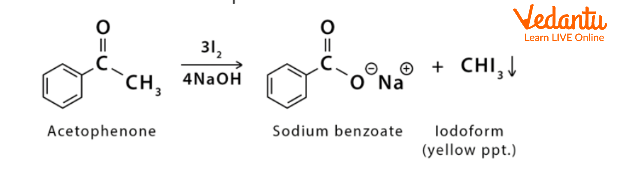
Conversion of Acetophenone Into Iodoform
The conversion of acetone into sodium acetate and chloroform
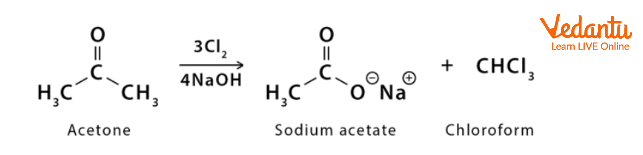
Conversion of Acetone Into Chloroform
Haloform Reaction of Ketones
Ketones having at least one methyl group linked to the carbonyl carbon atom can undergo haloform reactions. For example, the reaction of acetone in the presence of sodium hypohalite to produce the corresponding carboxylic acid and haloform is given below.
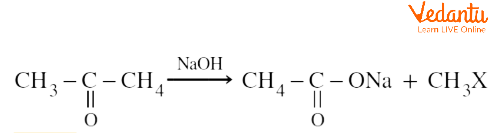
[X = Cl, Br, I]
Haloform Reaction of Ketones
Mechanism of Haloform Reaction
The mechanism of haloform reactions includes disproportionation of halogen to give hypohalite and halide, then the reaction of hypohalite with ketones or aldehydes, and at last, the formation of corresponding carboxylic acids and haloform. Taking the above example only, let’s understand the mechanism of the haloform reaction.
Firstly, the halogen gets dis-proportionates in the presence of hydroxide to give hypohalite and halide.
$B{{r}_{2}}+2O{{H}^{-}}\to B{{r}^{-}}+Br{{O}^{-}}+{{H}_{2}}O$
Ketone present in the reaction reacts with the hypohalite in three steps.
Step-1: The hydroxide ion takes out the hydrogen from alpha carbon and produces enolate. Further, the enolate and the halogen react to form halogenated ketone and halogens corresponding anion.
Step-2: There is a repetition of step 1 twice which yields a tri-halogenated ketone.
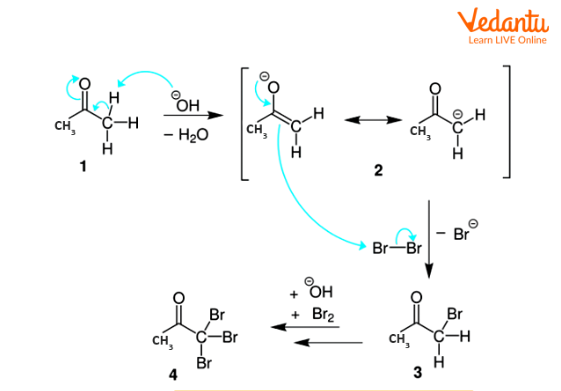
Mechanism of Formation of Halogenated Ketone
Step-3: The doubly bonded carbon atom which is attached to oxygen acts as an electrophile and is attacked by a hydroxide ion which acts as a nucleophile. This leads to the breakage of the carbon-oxygen bond, which makes the oxygen atom anionic and favourable for the formation of carboxylic acid. The carbon containing three halogens is displaced leaving the carboxylic acid behind. The carboxylic acid donates a proton to the tri-halomethyl anion to give the required haloform product.
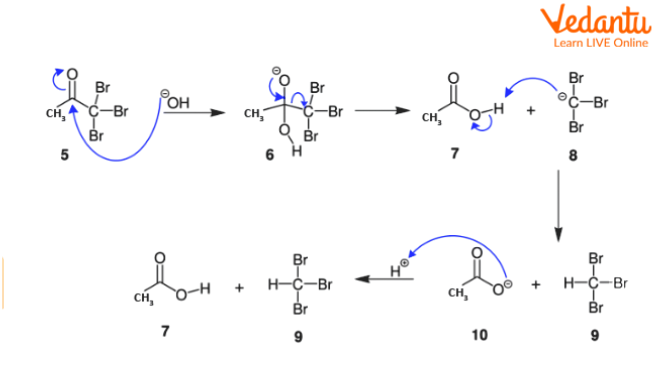
Mechanism of Formation of Carboxylic Acid and Haloform From Halogenated Ketone
Thus, this three-step haloform reaction mechanism leads to the formation of the required haloform and carboxylic acid. Fluoroform cannot be prepared by using a haloform reaction as the hypofluorite ion is highly unstable.
Haloform Reactions of Aldehydes
Aldehydes having at least one methyl group linked to the carbonyl carbon atom can undergo haloform reactions. But usually, aldehydes do not undergo haloform reactions. The only aldehyde which undergoes a haloform reaction is acetaldehyde. Acetaldehyde reacts with hypohalite to form carboxylate ions and haloform. For example, the reaction of acetaldehyde with iodine in the presence of hypohalite leads to the formation of a carboxylate ion and iodoform.
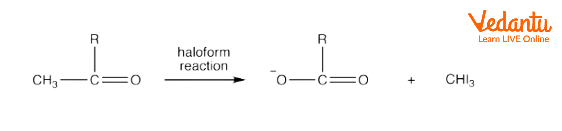
Haloform Reaction of Aldehyde
Acetaldehyde Formula
Acetaldehyde is one most important aldehyde which is produced on a large scale in industries. It is an organic compound and is given by the chemical formula CH3CHO. Acetaldehyde is a colourless liquid or gas and boils at room temperature.
Summary
Haloform reactions are those reactions that are used to produce haloforms such as chloroform $(CHC{{l}_{3}})$, bromoform $(CHB{{r}_{3}})$ or iodoform $(CH{{I}_{3}})$, and to convert acetyl groups to carboxyl groups. Fluoroform cannot be prepared by using a haloform reaction as the hypofluorite ion is highly unstable. This reaction is given by the aldehydes and ketones having at least one methyl group.
Ketones undergo haloform reactions in the presence of sodium hypohalite to produce the corresponding carboxylic acid and haloform. Aldehydes do not undergo haloform reactions except acetaldehyde. The reaction mechanism of the haloform reaction includes the disproportionation of halogen to give hypohalite and halide, then the formation of halogenated ketones or aldehydes and halogens corresponding to anion by the reaction of enolate and halogen. At last, the breakage of the carbon-oxygen bond makes the oxygen atom anionic and leads to the formation of carboxylic acid and haloform.
FAQs on Aldehydes and Ketones Haloform Reaction
1. What is a haloform reaction? Explain with the help of an example.
Reactions that are used to produce haloforms such as chloroform $(CHC{{l}_{3}})$, bromoform $(CHB{{r}_{3}})$ or iodoform $(CH{{I}_{3}})$, and carboxylic acid by reacting aldehydes or ketones with halogens such as chlorine, bromine, or iodine in the presence of hydroxide ions are called haloform reactions. Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom can undergo haloform reactions. For example, the reaction of acetone in the presence of sodium hypohalite to produce the corresponding carboxylic acid and haloform is given below.
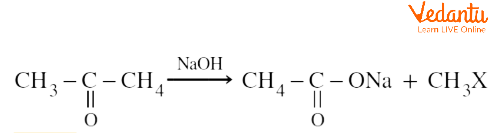
[X = Cl, Br, I]
Haloform Reaction of Ketones
2. Explain the three-step mechanism of the reaction of ketone with hypohalite.
Ketone present in the reaction reacts with the hypohalite in three steps:
Step-1: The hydroxide ion takes out the hydrogen from alpha carbon and produces enolate which reacts with halogen to form the halogenated ketone and halogen corresponding anion.
Step-2: There is a repetition of step 1 twice which yields a tri-halogenated ketone.
Step-3: There is a breakage of the carbon-oxygen bond which makes the oxygen atom anionic and favourable for the formation of carboxylic acid. The carbon-containing three halogens are displaced leaving the carboxylic acid behind which donates a proton to the tri-halomethyl anion to give the required haloform product.
























