




A Brief Overview of the Bohr and Haldane Effect
During respiration, oxygen and carbon dioxide are transported. For cellular functions, oxygen is carried from the lungs to the blood, while carbon dioxide is created by the cells and expelled from the body as a by-product. The pH influences the oxygen binding to molecules in red blood cells. When the pH drops, the oxygen-binding affinity decreases.
The Bohr Effect is the influence of pH on the oxygen affinity of haemoglobin molecules in the blood. Carbon dioxide can be carried in the blood, but only if oxygen and haemoglobin are present in a free form. The Haldane effect occurs when oxyhaemoglobin levels are low and carbon dioxide levels are high in the blood. By attaching separately to the haemoglobin molecule, oxygen and carbon dioxide are carried through the bloodstream.
The Haldane effect explains how oxygen concentrations affect haemoglobin's carbon dioxide affinity. In both circumstances, oxygen is the cause of the change in carbon dioxide levels. On the other hand, the Bohr Effect describes how carbon dioxide and hydrogen ions affect haemoglobin's oxygen affinity. Both the Bohr and Haldane effects function in combination to increase O2, unloading and uptake of excess CO2 and H+ in tissues, as well as O2 uptake and carbon dioxide unloading in the lungs.
What is the Haldane Effect?
In the lungs, oxygen mixing in the blood with haemoglobin helps in replacing the carbon dioxide from the blood that is carried out from tissues and this replaced carbon dioxide is eliminated from the lungs to the environment. The Haldane effect occurs when the blood contains low levels of oxyhaemoglobin and high levels of carbon dioxide.
Haldane Effect
Haemoglobin gets more acidic as it binds to oxygen during the Haldane effect. On expiration, this action causes carbon dioxide and H+ protons to eject into the alveoli. Expiration causes the Haldane Effect.
Factors Causing the Haldane Effect
High levels of carbon dioxide in the blood
Low levels of oxyhaemoglobin in the blood
Bohr’s Effect
The Bohr's effect is a link between the oxygen-binding activity of haemoglobin and the pH of the surrounding environment. In the context of low pH or high carbon dioxide, the Bohr's effect describes a decline in haemoglobin’s oxygen affinity. The haemoglobin tetramer is affected by both pH and carbon dioxide. The cause of the Bohr effect is a drop in pH.
The Bohr's effect is a shift to the right of the O2 dissociation curve caused by an increase in carbon dioxide or H+ ion concentration (reduction in pH) in the blood (i.e., causes oxygen to dissociate from haemoglobin). In the lungs, haemoglobin binds with four O2 molecules. The haemoglobin bound is 97% saturated. The Bohr effect causes a drop in the temperature in the lungs.
Physiological factors that can shift the oxygen curve to the right include:
An increase in temperature occurs during activities and even in healthy people.
Rise in DPG-2, 3-diphosphoglycerate levels.
Double Bohr Effect
It is a situation in which the Bohr effect is present in both the maternal and foetal circulations in the placenta. The unloading of oxygen is aided by a rise in the partial pressure of carbon dioxide in the maternal intervillous sinuses. On the foetal side of the circulation, a drop in the partial pressure of carbon dioxide aids the oxygen loading. The reciprocal exchange of oxygen with carbon dioxide is facilitated by the Bohr effect.
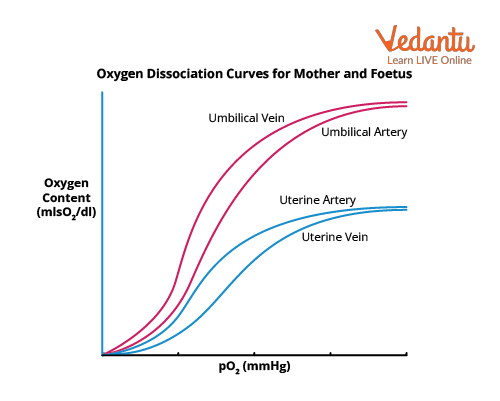
Oxygen Dissociation Curve
Cause of the Bohr Effect
A decrease in pH causes the Bohr effect, which diminishes haemoglobin’s affinity for oxygen. The Bohr effect is a link between the oxygen-binding activity of haemoglobin and the pH of the surroundings. When the pH falls, the concentration of H+ rises and vice versa. In the human body, where oxygen levels are low, carbon dioxide levels are high.
The citric acid cycle in tissues produces carbon dioxide by breaking down carbohydrates and lipids for energy. The enzyme carbonic anhydrase mixes carbon dioxide with water to produce bicarbonate and H+ ions. The pH falls when the H+ concentration rises. The oxygen-binding curve of haemoglobin is shifted to the right by an increase in H+ concentration, while the curve is shifted to the left by a reduction in H+ concentration.
What is Oxyhaemoglobin?
Haemoglobin coupled with oxygen is known as ‘oxyhaemoglobin’. Oxygen is delivered from the lungs to other tissues in the form of oxyhaemoglobin. When oxygen attaches to haemoglobin in the lungs, oxyhaemoglobin is generated. Oxygen reversibly binds to haemoglobin. RBCs contain the respiratory pigment haemoglobin (Hb). When the bulk of oxygen in the blood is coupled with haemoglobin, oxygen enters the RBC quickly and binds to it. There are 280 million Hb molecules in each RBC. Four oxygen molecules can be carried by each Hb molecule.
Hb + O2→ HbO2 (oxyhaemoglobin)
Factors Affecting the Formation of Oxyhaemoglobin
pH: The oxygen loading of haemoglobin is affected by pH via the Bohr effect. H+ or H3O+ (hydronium ion) is a waste product of cellular respiration that can bind to Hb and prevent it from transporting gases. The unloading of oxygen at the tissues is larger when the pH is low.
Temperature: Higher temperatures create a rightward shift in the oxyhaemoglobin dissociation curve. It indicates this because when the cells are undertaking a lot of cellular respiration (in the tissues), the temperatures are warmer and oxygen falls off of haemoglobin more quickly.
High carbon dioxide: The level of high carbon dioxide in the blood reduces the affinity of haemoglobin to oxygen. Carbon dioxide binds with Hb molecules and forms carbonic acid. These carbonic acids dissociate in blood and the product is hydrogen ions and bicarbonate ions. The increased concentration of H+ ions in the blood then increases the pH of the blood.
DPG: The oxygen loading of haemoglobin is affected by 2, 3-diphosphoglyceric acid (DPG) generated by RBCs. It reversibly binds to Hb to facilitate oxygen unloading.
The pH of Oxygenated Blood
The pH of normal blood is 7.35 to 7.45. Oxygen is carried through the blood as oxyhaemoglobin, and this blood is known as oxygenated blood. Haemoglobin has a high affinity for oxygen and binds with it when the pH is high.
Carbon Dioxide Dissociation Curve
The carbon dioxide dissociation curve depicts how the carbon dioxide concentration of blood changes as the partial pressure of carbon dioxide changes. The oxygen-haemoglobin dissociation curve is more linear and steeper than this one. There is no such thing as a plateau. As a result, the shunt has a negligible impact on carbon dioxide. When oxygen saturation is reduced, more carbon dioxide combines with haemoglobin, thereby promoting easier carbon dioxide removal from the tissues.
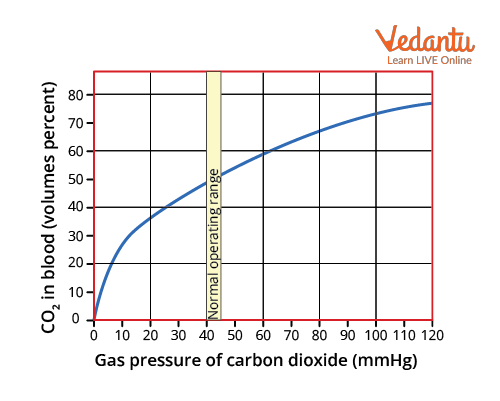
Carbon Dioxide Dissociation Curve
Conclusion
This article has given all the necessary details about the Bohr and Haldane effects with respect to the NEET syllabus. The questions added at the end of the article are frequently asked questions and are important from the exam point of view. Thus, the student can have a clear understanding of the types of questions that might come in the exam. For further readings and related articles, visit our website today!
FAQs on Bohr and Haldane Effect for NEET
1. One haemoglobin carries __________ molecules of oxygen.
Haemoglobin has a quaternary structure which is made up of four porphyrin rings, and its heme molecule is responsible for oxygen binding. Haemoglobin becomes saturated in the lungs where the oxygen pressure is high and binds with more and more oxygen as a result of the cooperation. Haemoglobin is made up of four units. One Fe atom is in the +2 state in each unit. A maximum of four oxygen molecules can be carried by each haemoglobin molecule. 1.34 ml of oxygen is transported by 1 gm of haemoglobin.
2. The Bohr Effect/Shift moves the oxygen saturation curve in which direction?
The Bohr Effect shifts the oxygen dissociation curve down and right, which is caused by an increase in carbon dioxide or H+ ion concentration (reduction in pH) in the blood (i.e., causes oxygen to dissociate from haemoglobin). When the oxygen dissociation curve is shifted to the right, less oxygen is bound to haemoglobin for a given partial pressure of oxygen. The oxygen supply to the tissues is influenced by a rightward change in the oxygen dissociation curve.
























