




Introduction to Chemical Bonding and Molecular Structure
In this chapter, we'll look at the structure of atoms, as well as the subatomic particles that make up atoms. To comprehend bond formation, one must first understand the overall characteristics of atoms' electronic structure, or the arrangement of electrons around the central nucleus.
This chapter is one of the most significant for competitive tests like NEET 2026, and mastering it will help you score well on the exams.
Important Topics of Chemical Bonding and Molecular Structure:
Chemical Bonding
Fajan’s rule
Lewis Dot Structures
Formal charge
Hybridization
Valence Bond Theory
Molecular Orbital Theory
VSEPR Theory
Important Definitions of Chemical Bonding and Molecular Structure:
Octet Rule:
The octet rule states that the atoms combine either by transfer of valence electron(s) or by sharing of valence electron(s) to form molecules by completing octet of electrons in their valence shell to attain the nearest noble gas configuration (hydrogen element completes its duplets to attain the configuration of helium).
Limitations of Octet Rule:
Incomplete Octet: The central atom of some molecules have incomplete octet i.e less than eight electrons in their valence shell. This is generally observed in the molecule of elements which has less than four valence electrons.
Example: BeCl2 (2 valence shell electrons in Be), BF3(3 valence shell electrons in B), LiH (1 valence shell electrons in Li).
Odd-electron Molecules: The molecules which are formed from the atom containing odd electrons in the valence shell.
Example: Molecules of nitrogen- Nitric oxide (NO) and nitrogen dioxide (NO2).
Extended Electron: The molecules of some elements contain more than eight electrons in the valence shell of central atoms. The elements of 3rd period and beyond have d orbitals which also participate in the chemical bonding.
Example: BrCl3 XeF2, PCl5, SF6
Noble Gas Compounds: Noble gases have complete octet but some noble gases such as xenon and krypton form compounds with fluorine and oxygen.
Example: XeOF2, XeF6 , KrF2, XeO3
Lewis Dot Structures:
G.N. Lewis proposed the notations to represent the elements with their valence electrons. The valence electrons are represented by the dot around the symbol of the element.
Example:

The molecules can also be represented by the lewis symbol notations in which:
Each atom shares a single electron to form an electron pair.
Each electron pair represents the bond.
The atoms combine to attain the noble gas configuration and each atom should have 8 electrons around it.
Example:
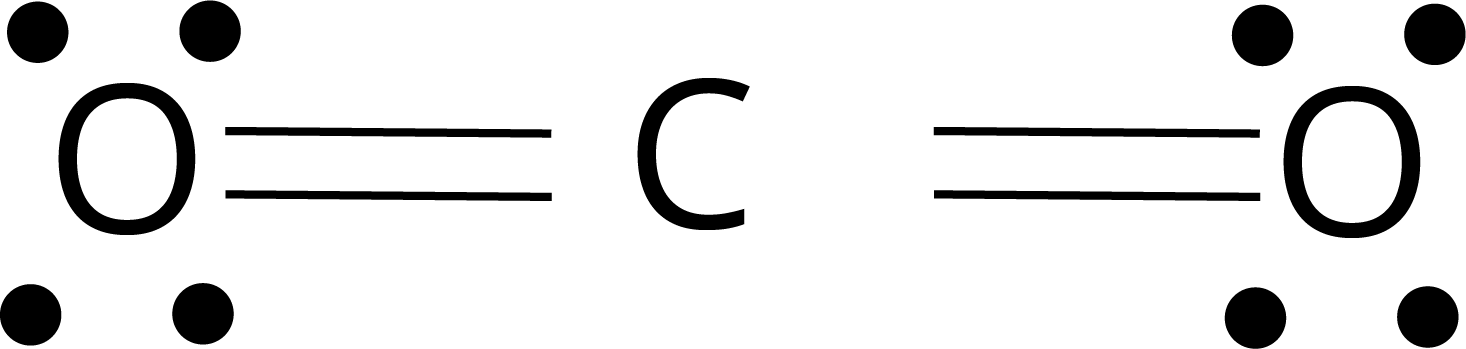
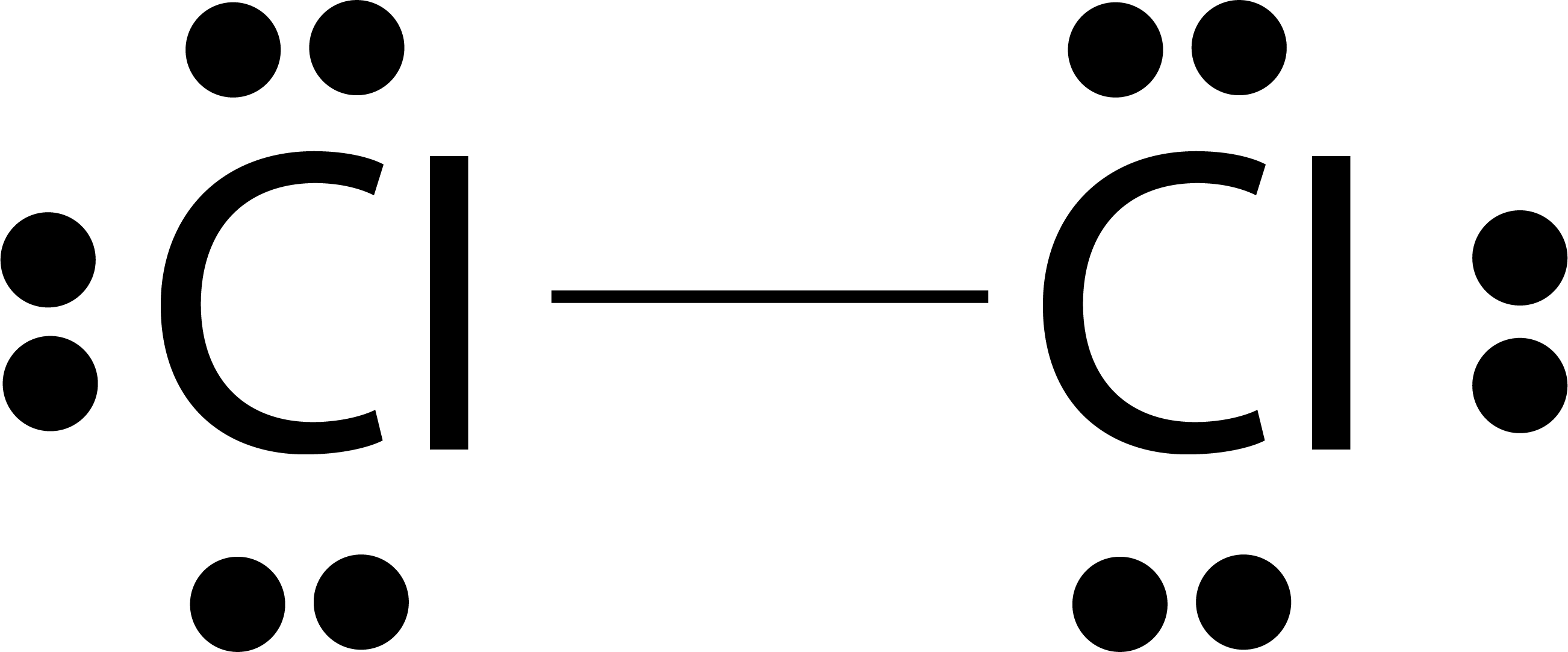
The single electron pair between two atoms represents a single covalent bond.
Example : H2O, NH3
The two electron pairs shared between two atoms represent the double bond.
Example : O2, CO2
The three electron pairs shared between two atoms represent the triple bond.
Example : CO, C2H2, N2
Covalent Bond:
The covalent bond is formed by sharing of electron (s) between two atoms. Atoms combine to become stable by attaining the nearest noble gas configuration.
When a single electron pair is shared between the atoms, a single covalent bond is formed, when two electron pairs are shared, a double covalent bond is formed and when three electron pairs are shared, a triple bond is formed.
Formal Charge:
The formal charge is the charge on each atom of the molecule or ion. The ions are represented by the net charge possessed by the ion whereas the formal charge is the charge possessed by each atom in the molecule or ion. In lewis structure the atoms in molecules or ions are represented by their formal charge.
The formal charge of an atom in a molecule or ion is given by the formula:
Formal Charge = V - N - ½ (B)
where, V: Total number of valence electrons present in the free atom
N: Total number of non-bonded electrons or lone pairs
B: Total number of bonded or shared electrons
The molecules in which the atoms possessing the smallest formal charge are considered to have the lowest energy and more stability.
Ionic or Electrovalent Bond:
Ionic or electrovalent bond is formed by the transfer of electron(s) from one atom to another to become stable by attaining the nearest noble gas configuration. The loss of electron(s) from the atom to form a positively charged ion is known as cation and the gaining of electron(s) forms a negatively charged ion known as anion. There is a strong electrostatic force of attraction between cations and anions, this force is the ionic bond.
Conditions for the formation of ionic bonds:
Low ionization enthalpy of cation
High electron gain affinity of anion
High lattice energy of ionic crystal
Ionisation Enthalpy: It is the amount of energy required to remove loosely bound electrons from an isolated gaseous state of an atom.
M(g) $\longrightarrow$ M+(g) + eー
Electron Gain Enthalpy: It is the amount of energy released when an electron is gained by the gas phase atom in its ground state.
X(g) + eー $\longrightarrow$ Xー(g)
Lattice Enthalpy: The amount of energy required to completely separate one mole ionic crystal into its constituent ions in gaseous state is known as lattice enthalpy of ionic crystal. More the lattice enthalpy of ionic crystal, more is the stability ion ionic crystal.
The factor on which the lattice enthalpy of ionic crystal depends:
Size of ions: Smaller the size of ions more will be the lattice enthalpy .
Charge on ions: Greater the charge on the ions stronger will be interionic attraction and hence more will be the lattice enthalpy.
Bond Parameters:
Bond Length:
It is the equilibrium distance between the nuclei of the two bonded atoms in a molecule.
The bond length of a covalent molecule is determined by the covalent radii of each bonded atom.
The bond length order:
Triple bond < Double bond < Single bond
Bond Angle:
It is the angle between the orbitals of molecules containing the bonded electron around the central atom.
It helps in determining the shape of the molecule.
It is expressed in degrees.
The factor that affects the bond angle:
Bond angle is proportional to the electronegativity of the central atom and inversely proportional to the electronegativity of the peripheral atoms.
Bond angle is proportional to the percentage of s character of the central atom and inversely proportional to the percentage of p character of the central atom.
Bond angle is proportional to bp-bp repulsion and inversely proportional to lp-bp and lp-lp repulsion.
Bond angle is proportional to size of peripheral atoms of a molecule.
Bond Enthalpy:
It is the amount of energy required to break one mole of a particular type of bond between the two atoms of a molecule in a gaseous state.
More the bond enthalpy, more will be the stability of the bond.
For polyatomic molecules, the bond enthalpy is the average or mean bond enthalpy of all the bonds in the molecule.
The factors that affect the bond enthalpy:
The chemical environment of the bond which is to be broken.
The more the number of bonds to break the more will be the bond enthalpy. The order of bond enthalpy:
Triple bond > Double bond > Single bond
Bond Order:
Bond order is the number of electron pairs or bonds present shared between two atoms of a molecule.
For single bond: one electron pair is shared between two atoms → bond order = 1
For double bond: two electron pairs are shared between two atoms → bond order = 2
For triple bond: three electron pairs are shared between two atoms → bond order = 3
Isoelectronic structures of molecules or ions have the same bond order.
Resonance Structure:
The resonance structure is the group of Lewis structures formed due to delocalization of electrons in a polyatomic molecule or ion.
Example: benzene and CO32-
The single lewis structure of the molecule could not explain the properties of some polyatomic molecules or ions, which is the implication that the molecule has resonance structures.
Polarity of Bonds:
The bond formed between two different atoms (or heteronuclear atoms) of the molecule has polarity in bond due to difference in electronegativity of different atoms and hence known as polar bond. Example: HCl, H2O
The polarity in bonds gives rise to the dipole moment in the molecule. The dipole moment in the molecule.
Dipole Moment:
The dipole moment is the product of charge (Q) and distance of separation between the atoms(r).
Dipole moment is a vector quantity and it is denoted by the symbol μ.
The unit of dipole moment is debye (D).
The resultant dipole moment of a molecule helps in determining the shape and polarity of a molecule.
Fajan’s Rule:
It states that the covalent bond has some partial ionic character whereas the ionic bonds have partial covalent character. The partial covalent character in ionic bond can be measured in terms of the following Fajan’s rule:
The smaller the size of cation and greater the size of the anion the more will be the covalent character in the ionic bond.
The cations with greater charge contribute to the more covalent character in the ionic bond.
Transition metal cations are more polarising than the cation with a noble gas configuration with the same charge and size as that of transition metal cations.
Valence Shell Electron Pair Repulsion (VSEPR) Theory:
This theory helps in the determining the shape of the molecule of covalent compounds on the basis of the repulsion of the lone pair and bond pairs in the free spatial arrangement around the central atom of the molecule. The postulates of the VSEPR are as follows:
Only valence-shell electron pairs around the central atom contribute to the shape of the molecule.
The electron pair repels each other as these are negatively charged due to their negatively charged electron clouds.
Electron pairs occupy the spherical space around the central atom such that they are at maximum distance from each other and have minimum repulsion between them.
Multiple bonds are treated as a single electron pair.
The repulsion order of bond pair(bp) and lone pair(lp) is:
lp-lp > lp-bp > bp-bp
Hybridisation:
It is the mixing of two orbits of nearly different energy levels to get the same number of orbitals of the equivalent energy and shape.
For example: The 1s and 2p orbitals of the same shell hybridize to give 3 orbitals of sp2 hybridisation.
Number of hybrid orbitals can be determined by the formula:
Number of hybrid orbitals (X) = ½ (Total valence electron of central atoms) - Total number of monovalent atoms - charge on cation + charge on anion.
Or
Number of hybrid orbitals (X) = number of bond pairs + number of lone pairs on central atoms
Hybridisation helps in the determination of geometry of molecules. The bond pairs and lone pairs both are responsible for the geometry of the molecule whereas only bond pairs are responsible for the shape of the molecule.
Table: Hybridisation corresponding to the number of hybrid orbital and geometry of molecule
Valence Bond Theory:
This theory states that, when the two atoms are brought closer to each other, there are some attractive forces as well as some repulsive forces acting between the two atoms. When the bond is formed, the net attractive force is balanced by the repulsive force.
Let us consider two hydrogen atoms which contain nuclei NA and NB and electrons in the valence shell as eA and eB. When the two atoms are brought closer, the attractive forces- NA - eA and NB - eB ; also NA - eB and NA - eA act between the atoms whereas the repulsive forces- NA -NB and eA and eB also act at the same time. A stage is reached when the net attractive force is balanced by net repulsive force, and the bond between two hydrogen atoms is formed.
Types of Covalent Bond:
There are two types of covalent bonds:
Sigma (σ) bond: This type of covalent bond is formed by the head-on overlapping of two atomic orbitals along the internuclear axis.
s-s overlapping: The two half filled s-orbitals of the two atoms overlap along the internuclear axis.

s-p overlapping: The half filled s-orbital of one atom overlaps with the half filled p-orbital of another atom along the internuclear axis.
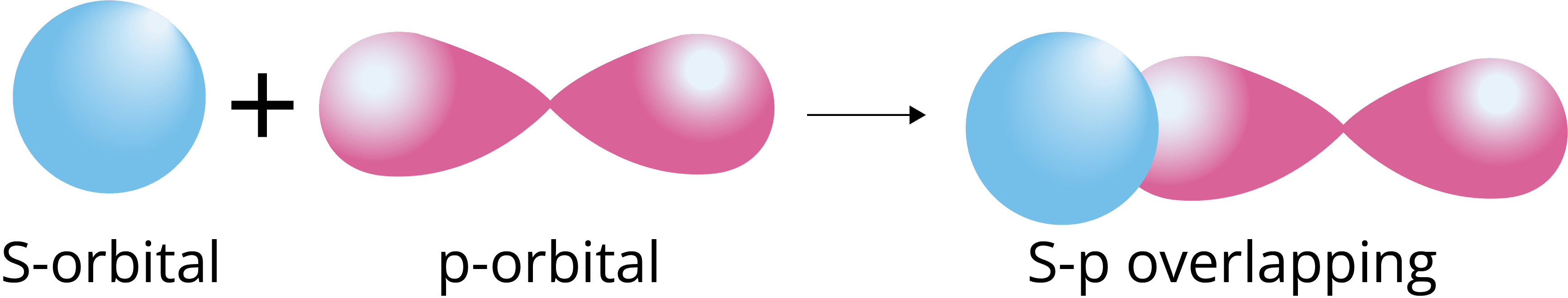
p-p overlapping: The half filled p-orbital of one atom overlaps with the half filled p-orbital of another atom along the internuclear axis.
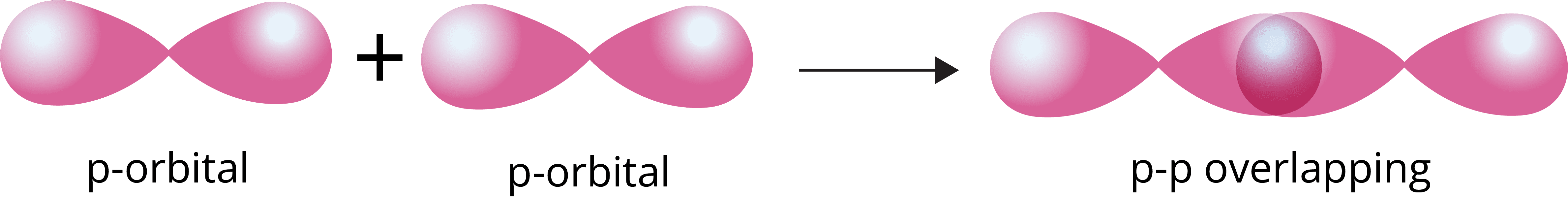
Pi (π) bond: This type of covalent bond is formed by the lateral or sidewise overlapping of two atomic orbitals perpendicular to the internuclear axis.
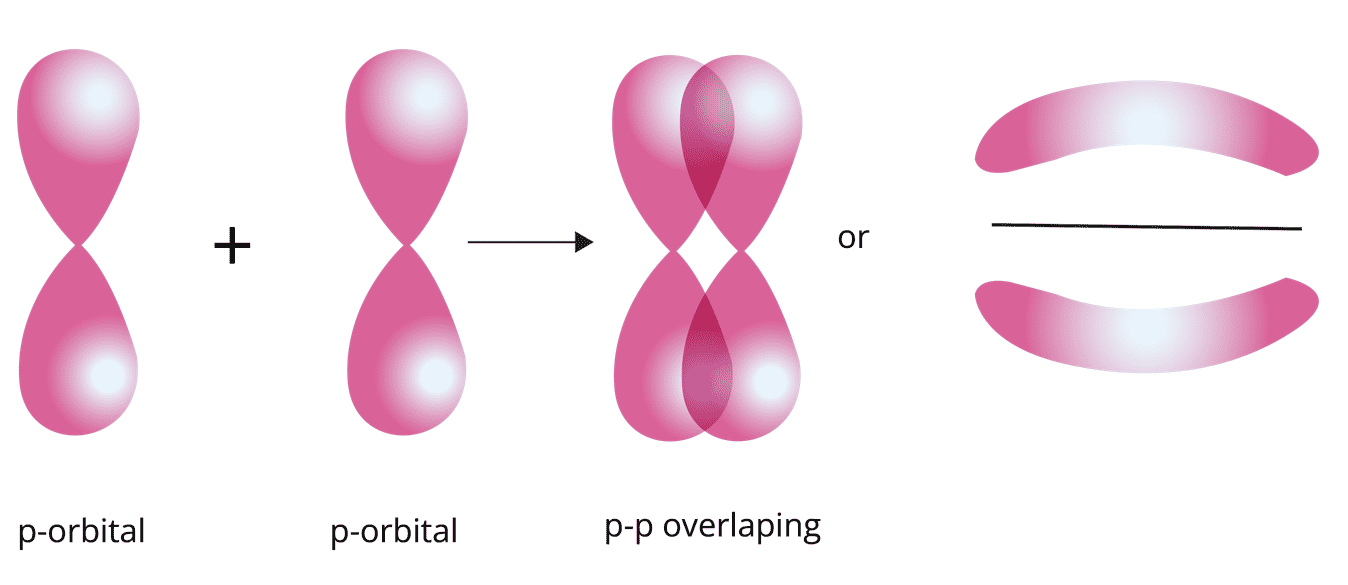
Strength of Sigma (σ) and Pi (π) Bond:
Sigma (σ) bond is formed by the head-on overlapping whereas the pi (π) bond is formed by sideways overlapping of the orbital.
Extent of overlapping is greater in the sigma (σ) bond than in pi (π) bond. Therefore, the strength of the sigma (σ)bond is more than the pi (π) bond.
In multiple bonds between the atoms, pi bonds are formed in addition to the presence of sigma bonds.
Molecular Orbital Theory (MOT):
According to MOT, the atomic orbital of the atoms combine by linear combination of atomic orbital (LCAO) to form the molecular orbitals.
The addition of an atomic orbital gives rise to the bonding molecular orbital whereas the subtraction of atomic orbitals gives rise to the antibonding molecular orbital. The anti bonding MOs are represented by (*) and they have more energy than bonding MOs.
ψMO = ψA ± ψB
Bonding MOs: σ 2s, σ2pz, π2py, π2px
Antibonding MOs: σ* 2s, σ*2pz, π*2py π*2px
Electronic Configuration of Molecule:
The distribution of electrons in the orbital of molecules in the increasing order of energy is known as the electronic configuration of molecules.
The electronic configuration of molecules which have more than 14 electrons such as O2 has 16 electrons, have electronic configuration as follows:
σ 1s2, σ* 1s2, σ 2s2, σ* 2s2, σ2pz2 (π2py2= π2px2) , (π*2py1 =π*2px1)
The electronic configuration of molecules which have equal to or less than 14 electrons such as N2 has 14 electrons, have electronic configuration as follows:
σ 1s2, σ* 1s2, σ 2s2, σ* 2s2, (π2py2= π2px2), σ2pz2
Bond Order:
Bond order is half of the difference of the electrons in bonding orbital and antibonding orbitals.
B.O. = ½ (Nb - Na)
where, Nb = Total number of electrons in bonding orbital
Na = Total number of electrons in antibonding orbital
Example: O2
B.O. (O2)= (10 - 6)/2 = 4/2 = 2
The O2 molecule has 2 bonds between each O atom.
Magnetic Properties:
The molecule is said to be diamagnetic if all the electrons in the molecular orbitals are paired. The molecule is said to be paramagnetic if the electron has atleast one unpaired electron in the molecular orbital.
Example: O2 molecule is a paramagnetic in nature as it has 2 unpaired electrons in each in the π*2py1 and π*2px1
Spin only magnetic moment can be calculated by the formula:
μs = $\sqrt{n(n + 2)}$
where, n : Number of unpaired electrons
Hydrogen Bonding:
When the highly electronegative elements such as fluorine, nitrogen and oxygen, are bonded covalently with the H atom, the electron of hydrogen atom is slightly shifted towards the highly electronegative atom, which develops a partial positive charge on H atom. The partial positive charged hydrogen atom is attracted towards the electronegative atom causing hydrogen bonding.
There are two types of hydrogen bonding:
Intermolecular Hydrogen Bonding: The hydrogen bonding is formed between two molecules of same or different compounds.
Example: H2O, HF
Hδ+-Fδ-……Hδ+-Fδ-…Hδ+-Fδ-…Hδ+-Fδ-
Intramolecular Hydrogen Bonding: The hydrogen bonding is formed when the highly electronegative (O, F, N) atom in the molecule is attracted to the hydrogen atom bonded to the electronegative atom (O, N, F) within the molecule.
Example: o-nitrophenol
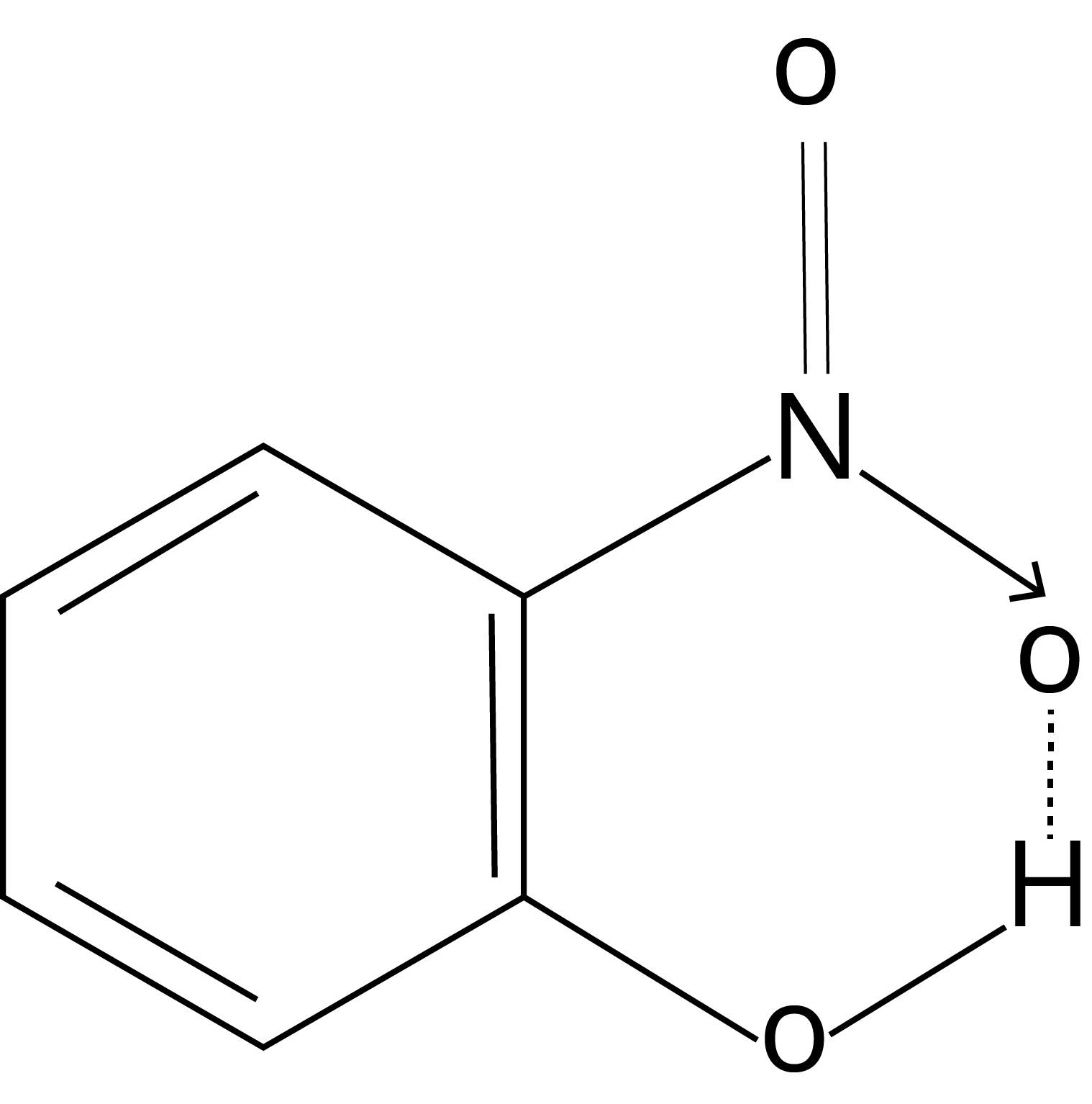
Solved Examples from the Chapter
Example 1: The bond order and magnetic behaviour of O2– ion are respectively :
1.5 and paramagnetic
1.5 and diamagnetic
2 and diamagnetic
1 and paramagnetic
Solution: (a) 1.5 and paramagnetic
Electronic configuration O2– (17 electrons):
σ 1s2, σ* 1s2, σ 2s2, σ* 2s2, σ2pz2 (π2py2= π2px2) , (π*2py2 = π*2px1)
B.O. (O2– )= (10 - 7)/2 = 3/2 = 1.5
The O2– molecule has 1 unpaired electron in the π*2px1, so it is paramagnetic in nature
Key Points:
O2– molecule has 17 electrons
B.O. = ½ (Nb - Na)
The molecule is said to be paramagnetic if there is alteast one electron in the molecular orbital.
Example 2: The correct shape and I — I — I bond angles respectively in I3– ion are :
Linear; 180°
T-shaped; 180° and 90°
Trigonal planar; 120°
Distorted trigonal planar; 135° and 90°
Solution: (a) The shape of I3– ion:
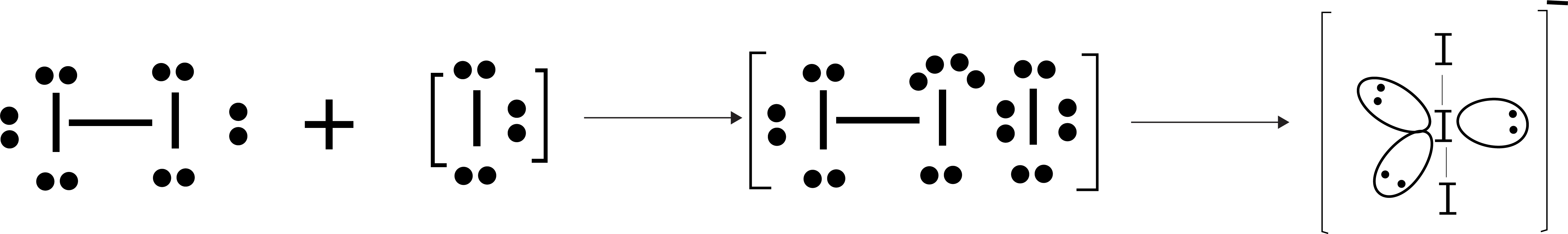
Central I atom has 3 lone pairs and 2 bond pairs, therefore, the hybridisation will be sp3d, so the geometry of the molecule will be trigonal bipyramidal, the two peripheral I atoms will occupy the axial position and have 180 degree between I — I — I .
Key Points:
Hybridisation = bond pairs + lone pairs
In hybridisation, geometry of the molecule is represented, whereas in the shape of the molecule only bond pairs are considered.
Solved Questions from the Previous Year Question Papers
Question 1: The compound that has the largest H-M-H bond angle (M = N, O, S, C) is:
CH4
H2S
NH3
H2O
Solution: (a)
The hybridisation of CH4, H2S, NH3, H2O is sp3, so the geometry of the molecule will be tetrahedral, more the lone pairs less will be the bond angle.
Therefore, the compound CH4 has the largest H-M-H bond angle.
Question 2: The shape or structure of [XeF5]– and XeO3F2, respectively, are
Pentagonal planar and trigonal bipyramidal
Trigonal bipyramidal and pentagonal planar
Octahedral and square pyramidal
Trigonal bipyramidal and trigonal bipyramidal
Solution: (a) [XeF5]– : 5 bond pairs + 2 lone pairs ⟶ sp3d3 hybridisation ⇒ Pentagonal planar shape
XeO3F2: 5 bond pairs + 0 lone pairs ⟶ sp3d hybridisation ⇒ Trigonal bipyramidal shape
Question 3: Among the following the maximum covalent character is shown by the compound :
(a) AlCl3
(b) MgCl2
(c) FeCl2
(d) SnCl2
Solution: (a) AlCl3 has the maximum covalent character in the given ionic compounds, due to maximum charge Al3+while all other cations have +2 charge.
According to fajan’s rule of covalent character in ionic bond, the cation with small size and greater charge has the maximum covalent character.
Practice Questions
Question 1: HF has highest boiling point among hydrogen halides, because it has :
lowest dissociation enthalpy
strongest hydrogen bonding
lowest ionic character
strongest van der Waals' interactions
Answer: (b) strongest hydrogen bonding
Question 2: The type of hybridisation and number of lone pair (s) of electrons of Xe in XeOF4, respectively,
are:
sp3d and 2
sp3d2 and 2
sp3d and 1
sp3d2 and 1
Answer: (d) sp3d2 and 1
Conclusion
It is concluded from various theories that the structure of atoms is the basis for the formation of bonds in the molecule as the valence shell electrons participate in the bond formation either by sharing of electrons or transfer of electrons. The covalent bond which is formed by sharing electrons has some ionic character whereas the ionic bond which is formed by electron transfer has some covalent character which can be measured by fajan’s rule.
Chemical bonding and molecular structure is collectively an important topic for the NEET competitive examination. One can find all types of questions- easy, medium and difficult form this chapter in the examination. This article covers all the major and important topics for the examination point of view.
NEET Chapter - Chemical Bonding and Molecular Structure

 Share
ShareFAQs on NEET Chapter - Chemical Bonding and Molecular Structure
1. Is the chemical bonding chapter important for NEET?
Yes, chemical bonding is an important chapter for the NEET exam as the questions appearing in the examination paper are of easy level in terms of difficulty. The 2-3 questions from this chapter come in the exam papers.
2. What are the important topics in chemical bonding for NEET?
The important topics in chemical bonding for NEET are:
Lewis dot structures
Fajan’s rule
Valence Shell Electron Pair Repulsion (VSEPR) theory
Resonance Structure
Molecular Orbital Theory (MOT)
Magnetic Properties of molecules
Dipole Moment
Hydrogen bonding
3. Is chemical bonding there in NEET?
Yes, chemical bonding is an important topic even for NEET as it is the basis for the important chapters and topics of chemistry.




















 Watch Video
Watch Video


