




What is an Electron Transport System?
The electron transport system occurs in the cristae of the mitochondria, where a series of cytochromes and coenzymes exist. These cytochromes and coenzymes go about as transporter atoms and move particles. They accept high-energy electrons and pass the electrons to the following atom in the system. At key proton-pumping sites, the energy of the electrons transports protons across the layer into the outer membrane of the mitochondrion.
Each NADH particle is exceptionally energetic, which represents the exchange of six protons into the outer compartment of the mitochondrion. Each FADH2 atom represents the exchange of four protons. The progression of electrons is similar to that occurring in photosynthesis. Electrons pass from NAD to FAD, to different cytochromes and coenzymes; and in the long run, they lose a lot of their energy. In cellular respiration, the last electron acceptor is an oxygen molecule. In their energy-depleted condition, the electrons join with an oxygen molecule. The electron-oxygen combination then responds with two hydrogen particles (protons) to shape a water particle (H2O).
Mitochondria
Mitochondria are organelles inside eukaryotic cells that produce adenosine triphosphate (ATP), the fundamental energy atom utilised by the cell.
Consequently, the mitochondrion is at times alluded to as the "Powerhouse of the cell". Mitochondria are found in all eukaryotes, which are living things that are not bacteria or archaebacteria.
It is believed that mitochondria emerged from once free-living bacteria that were incorporated into cells.
Mitochondria are a double membrane organelle, an outer membrane layer and an inner membrane.
The inner membrane is a lot bigger than the outer membrane, yet is associated with internal folding called cristae.
In the middle between the internal and outer membrane, there is an intermembrane space and the space within the inner membrane is known as the matrix, which contains enzymes and different reagents.
The outer membrane is permeable to different particles since it contains a mitochondrial porin, which is a pore-shaping protein.
The inner membrane is impermeable to most ions and polar atoms and only permeable to ATP, pyruvate and citrate due to metabolite carriers.
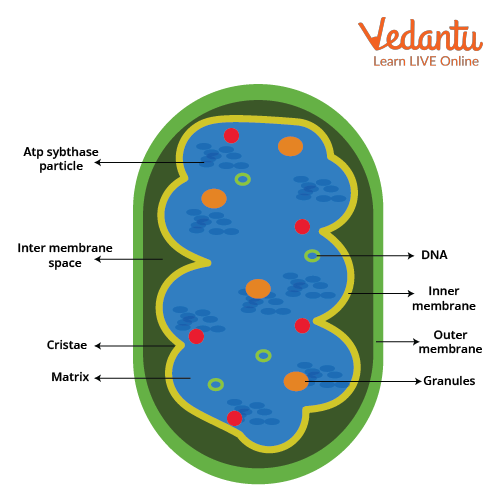
Mitochondria
Electron Transport Chain
An electron transport chain is a group of proteins that move electrons through a membrane inside mitochondria to frame a gradient of protons that drive the production of adenosine triphosphate (ATP).
The electrons move from one carrier to another, down an ideal energy gradient to the last electron acceptor, O2.
The ideal energy gradient is because of the way that electrons move towards parts with a more positive reduction potential and in this manner a higher affinity for oxygen.
As the electron flow happens, protons are streaming across the inner membrane into the intermembrane space.
The carrier proteins are arranged in an increasing positive redox potential manner.
Each carrier gets reduced as it accepts an electron and subsequently reduced when it loses the electron to the next carrier and electrons fall to lower energy states.
Electron transfer chain reaction is an exergonic process.
The energy that is released by electrons is used to pump hydrogen atoms outside the inner membrane i.e., the intermembrane space against the concentration gradient.
This H+ is used by the ATP synthase to produce ATP.
Below is the representation of the electron transport chain diagram.
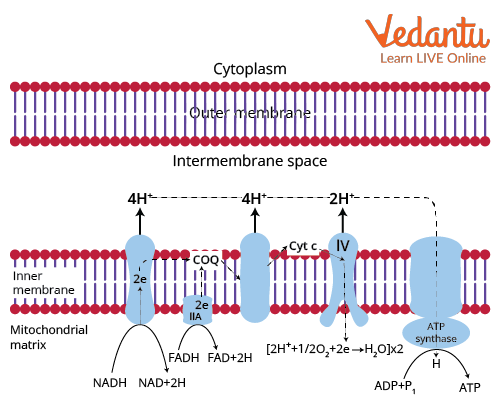
Electron Transport System Diagram
Components of Electron Transport System
At the point when electrons stream down the energy gradient from NADH to O2, the four protein complexes that catalyse the redox reaction are:
Complex I = NADH-Q reductase complex
Complex III = Cytochrome c reductase complex
Cyt C = Cytochrome c
Complex IV = Cytochrome c oxidase complex
At the point when electrons stream down the energy gradient from FADH2 to O2, the four protein complexes that catalyse the redox reactions are:
Complex II = succinate dehydrogenase
Complex III= Cytochrome c reductase complex
Cyt C = Cytochrome c
Complex IV = Cytochrome c oxidase complex
Electrons from FADH2 enter the electron transport chain at the fourth protein complex, succinate-Q reductase.
This protein complex contains succinate dehydrogenase which was liable for creating FADH2 from the reactions converting succinate into fumarate.
Since FADH2 electrons enter the electron transport chain later in the cycle, they pump fewer H+ particles to the intermembrane space (protein complex II doesn't pump H+ particles), and that implies less ATP is produced.
Four Complexes of Electron Transport System
Complex 1-NADH-Q Oxidoreductase:
It includes enzymes comprising iron-sulphur and FMN.
Here two electrons are carried to the first complex aboard NADH.
FMN is derived from vitamin B2.
Complex 2-Succinate-Q Reductase:
FADH2 that isn't gone through complex 1 is gotten straightforwardly from complex 2.
Compound ubiquinone (Q) combines first and second complexes.
The Q atom is dissolvable in water and moves uninhibitedly in the hydrophobic core of the membrane.
In this stage, an electron is conveyed straightforwardly to the electron protein chain.
The quantity of ATP obtained at this stage is straightforwardly relative to the number of protons that are pumped across the inner layer of the mitochondria.
Complex 3-Cytochrome c Reductase:
This complex includes Fe-S protein, Cytochrome b, and Cytochrome c proteins.
Cytochrome proteins comprise the heme group.
This complex is liable for pumping protons across the membrane.
It likewise passes electrons to the cytochrome c where it is moved to the fourth complex of enzymes and proteins.
Here, Q is the electron giver and Cytochrome C is the electron acceptor.
Complex 4-Cytochrome c Oxidase:
This complex involves cytochrome c, a and a3.
There are two heme groups and every one of them is available in cytochrome c and a3.
The cytochromes are answerable for holding oxygen atoms among copper and iron until the oxygen content is totally diminished.
In this stage, the decreased oxygen picks two hydrogen particles from the surroundings to make water.
Inhibitors of Electron Transport System
Rotenone and Amytal block electron transfer from Complex I to CoQ.
Cyanide and Azide inhibit ETS by reacting with ferric from heme.
Carbon monoxide inhibits the ferrous form.
2, 4-DNP causes uncoupling of electron transport.
Conclusion
This article gives an insight into the electron transport system and how the electrons flow from one complex to another. It also discusses which complex gets oxidised and reduced. Besides, we learn that oxygen acts as a final acceptor and ATP is produced at the end of the transfer of electrons. This article covers the most important topic which is frequently asked in the examination.
FAQs on Electron Transport System: Summary for NEET
1. What are the most important questions asked from the Electron Transport System?
Examination frequently asks questions about the sequence of the different complexes in this system, which complex is responsible for donating electrons at the terminal, which acts as the final acceptor, and which molecule is formed in the end. These are one of the most important and repeated questions asked in the examination. Students shouldn’t avoid the electron transport system, as questions are asked almost every year from this. The electron transport chain reaction and electron transport chain diagram should be seen by the students properly.
2. In which part of the cell does the electron transport system take place?
The location of occurrence of different processes in biology is the most confusing and most frequently asked question in the examination, a student is advised to make a separate list of different processes and organelles in which they occur to avoid further confusion and gain more clarity in the concept.
A student is advised to look through the diagrams and practice the steps of the ETS. They should make mind maps and pathway diagrams for a clear and better understanding of the concept.
























