




Hydrocarbons Class Notes
Hydrocarbons play an important role in our everyday lives. You should be familiar with the words "LPG" and "CNG'' when it comes to fuels. CNG stands for compressed natural gas, whereas LPG stands for liquefied petroleum gas. Another word in use these days is 'LNG' (liquified natural gas). This is also a fuel that is made from natural gas liquefaction. Fractional distillation of petroleum located beneath the earth's crust yields petrol, diesel, and kerosene oil. Coal gas is made by distilling coal in a damaging manner. During the drilling of oil wells, natural gas is discovered in the upper strata.
In this hydrocarbon notes for neet, we have discussed all the important concepts: properties of hydrocarbon, classification of hydrocarbon, and reaction of hydrocarbon, that will be helpful in Neet examination.
Important Topics of Hydrocarbons
Alkane
Alkene
Cyclic Haloalkane
Anti-Markovnikov's Rule
Pyrolysis
Decarboxylation
Wurtz Reaction
Markonikoff’s Rule
Aromatic Hydrocarbon
Important Definition of preparation properties and reactions of Hydrocarbons
What is Hydrocarbon Explain the Classification of Hydrocarbons?
The term 'hydrocarbon' is self-explanatory and refers to just carbon and hydrogen-based substances. Polymers such as polythene, polypropene, and polystyrene are also made with hydrocarbons. Paint solvents are made up of higher hydrocarbons. They're also employed as raw materials in the production of a variety of colours and pharmaceuticals. As a result, you can appreciate the significance of hydrocarbons in your daily life.
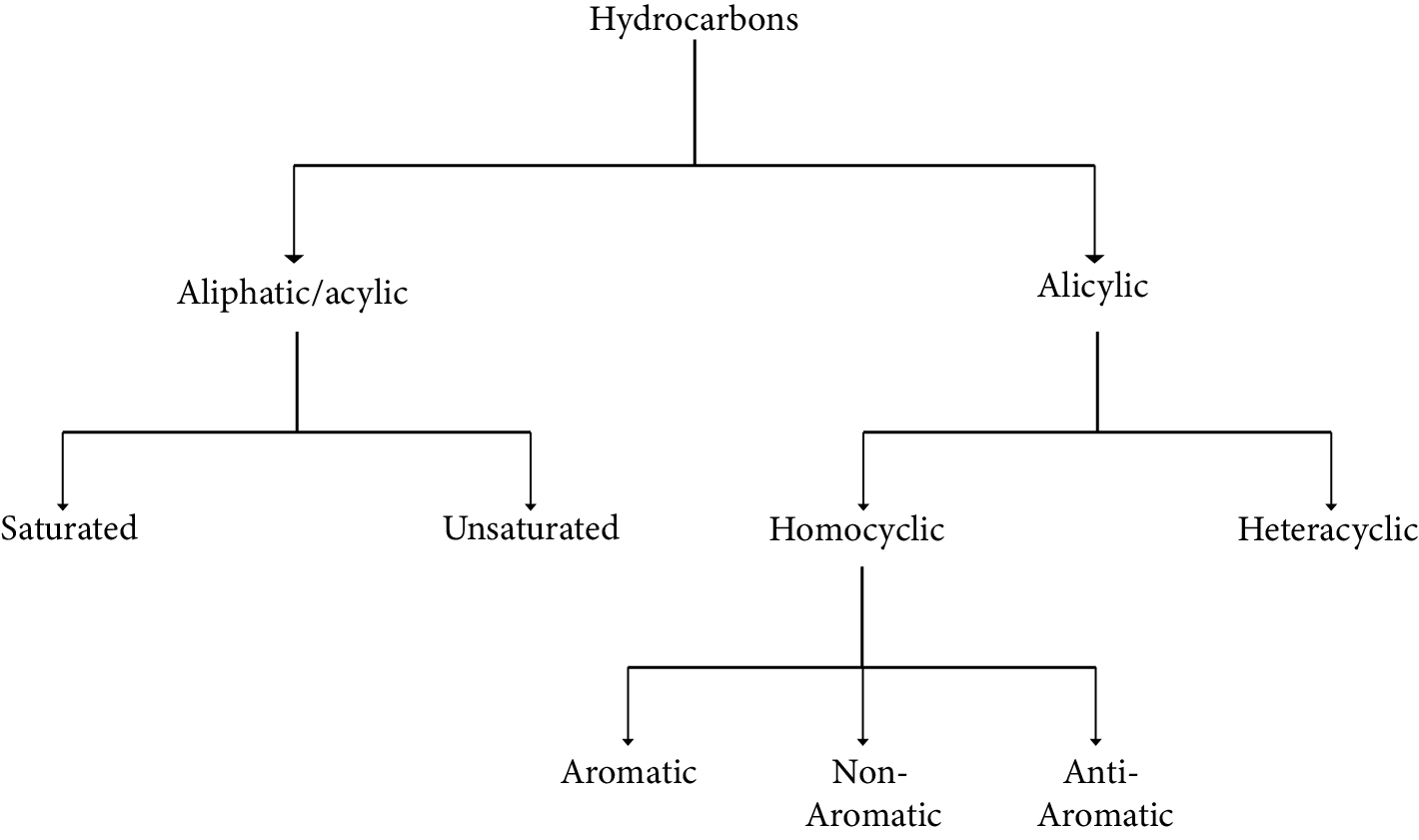
Classification of Hydrocarbons
Hydrocarbons come in a variety of forms. Carbon-carbon bonds can be categorized into three categories based on the types of carbon-carbon bonds present.
Saturated Hydrocarbons
Aromatic hydrocarbons
Unsaturated hydrocarbons
Saturated Hydrocarbons
Carbon-carbon and carbon-hydrogen single bonds are found in saturated hydrocarbons. Alkanes are formed when distinct carbon atoms are linked together to form an open chain of carbon atoms with single bonds.
Unsaturated Hydrocarbons
Carbon-carbon multiple bonds – double bonds, triple bonds, or both – are found in unsaturated hydrocarbons.
Aromatic Hydrocarbons
Aromatic hydrocarbons are a sort of cyclic compound that contain pi electrons arranged in an alternate manner.
Chart of Hydrocarbons
Physical Properties of Hydrocarbons
Hydrocarbons are nonpolar in nature.
Hydrocarbons with lower numbers of carbon are gasses, while those with higher numbers of carbon are in solid or liquid state.
They generally do not possess odor except aromatic hydrocarbons.
With increasing molecular mass and surface area, the boiling point rises steadily.
Chemical Properties of Hydrocarbon
Hydrocarbons are generally non reactive in nature due to their nonpolar nature. But, it gives some reactions. The chemical reactions of hydrocarbons are given below:
1. Halogenation reaction of hydrocarbon
CH4 + Cl2 → CH3Cl + HCl (in presence of sunlight)
CH3Cl + Cl2 +→ CH2Cl2 + HCl (in presence of sunlight)
CH2Cl2 + Cl2 → CHCl3 + HCl (in presence of sunlight)
CHCl3 + HCl → CCl4 + HCl (in presence of sunlight)
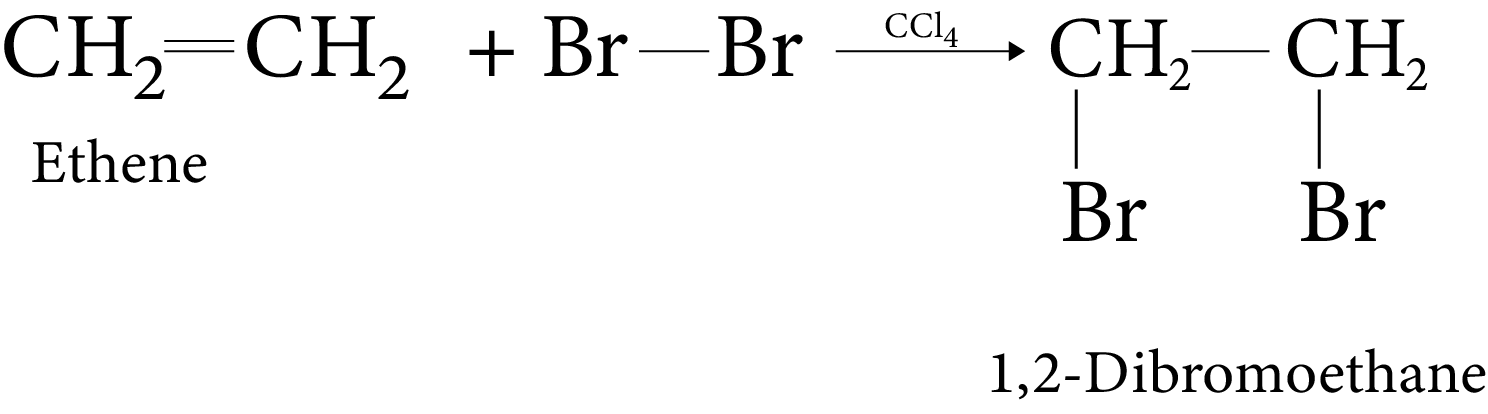
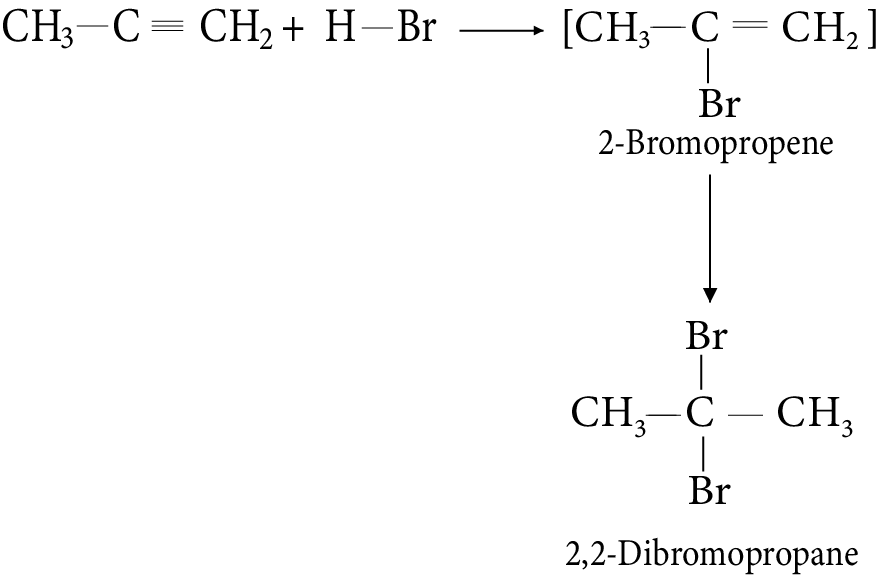
It is found that the rate of reaction of alkanes with halogens (X) is: F2 > Cl2 > Br2 > I2.
Rate of replacement of hydrogens (H) of alkanes (R) is: 3° > 2° > 1°.
2. Combustion
CH4 + 2O2 → CO2 + 2H2O + energy (Complete combustion)
CH4 + 2O2 → C + 2H2O (Incomplete combustion)
3. Controlled oxidation
2CH4 + O2 + Cu → CH3OH (at 523K and 100atm)
CH4 + O2 + Mo2O3 → HCHO + H2O
2C2H6 + 3O2 + (CH3COO)2Mn→ 2CH3COOH + 2H2O
Normally, alkanes resist oxidation, however potassium permanganate can oxidize alkanes with a tertiary H atom to the equivalent alcohols.
(CH3)3C-H + KMnO4 → (CH3)3C-OH
4. Isomerization
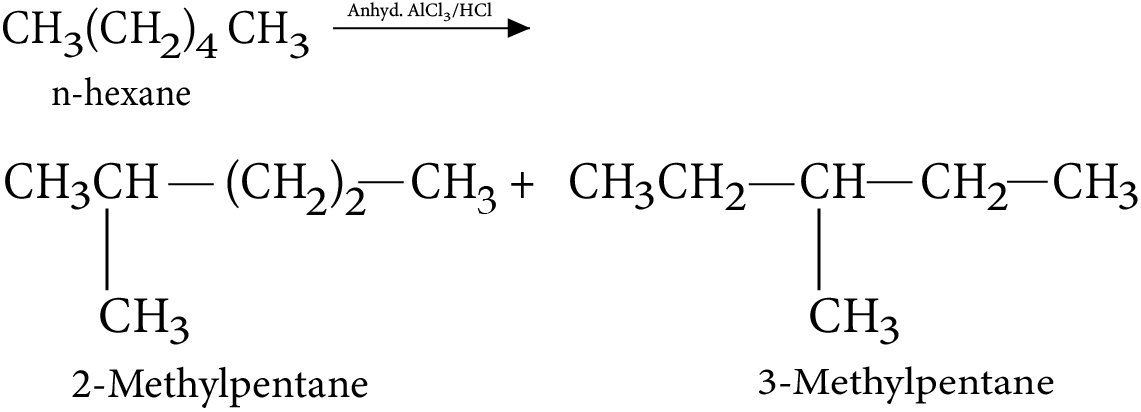
5. Aromatization
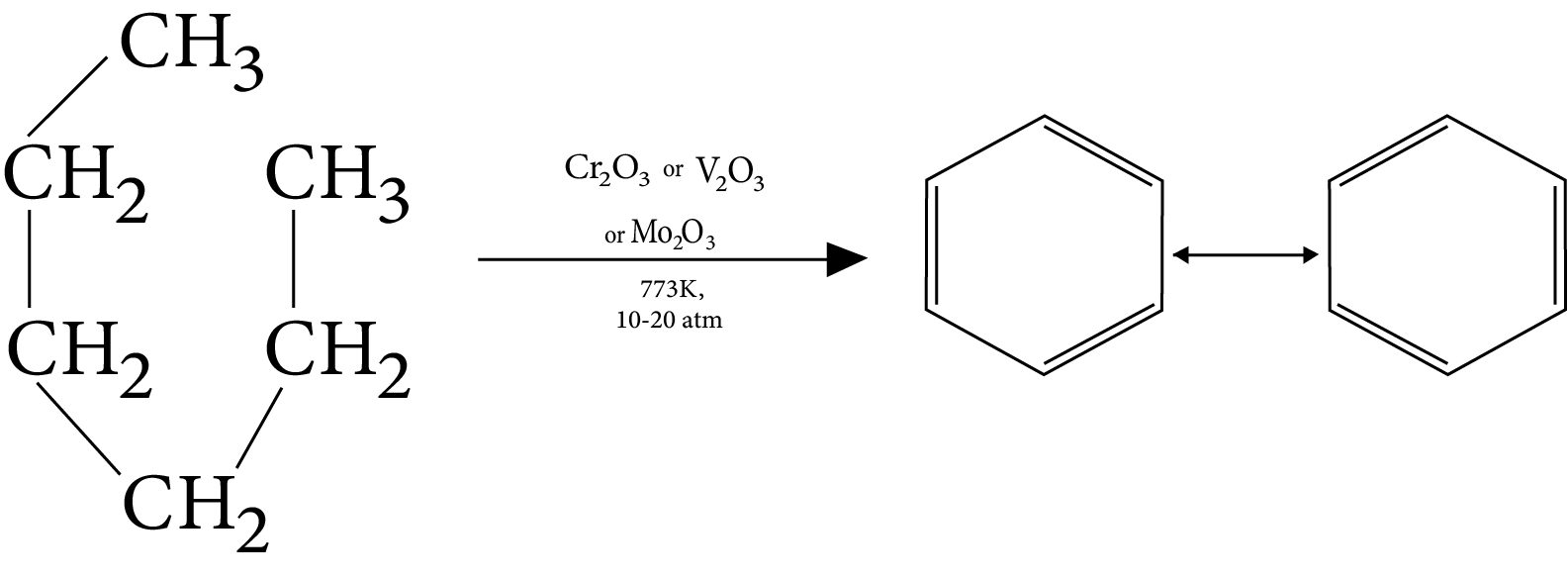
6. Pyrolysis
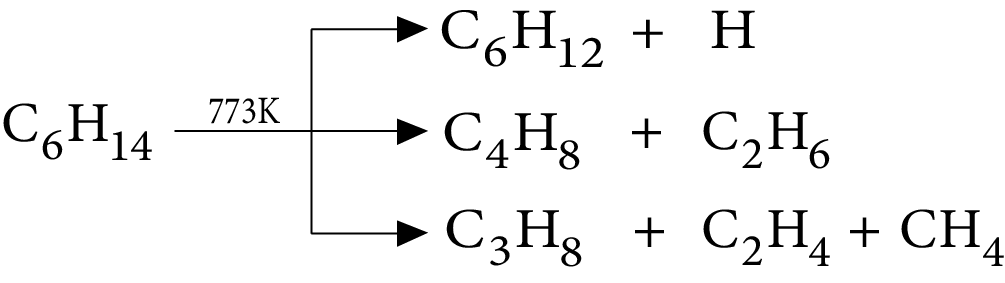
7. Addition of Hydrogen Halide
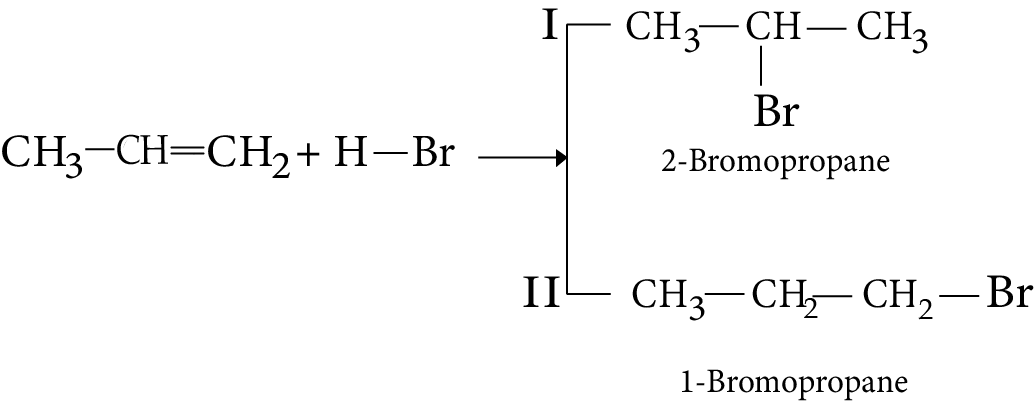
8. Addition of water
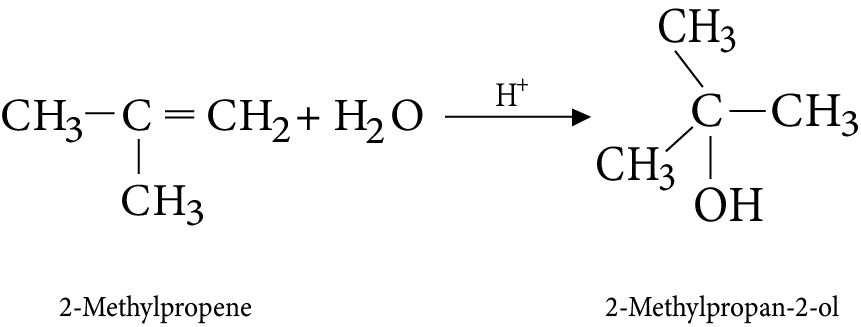
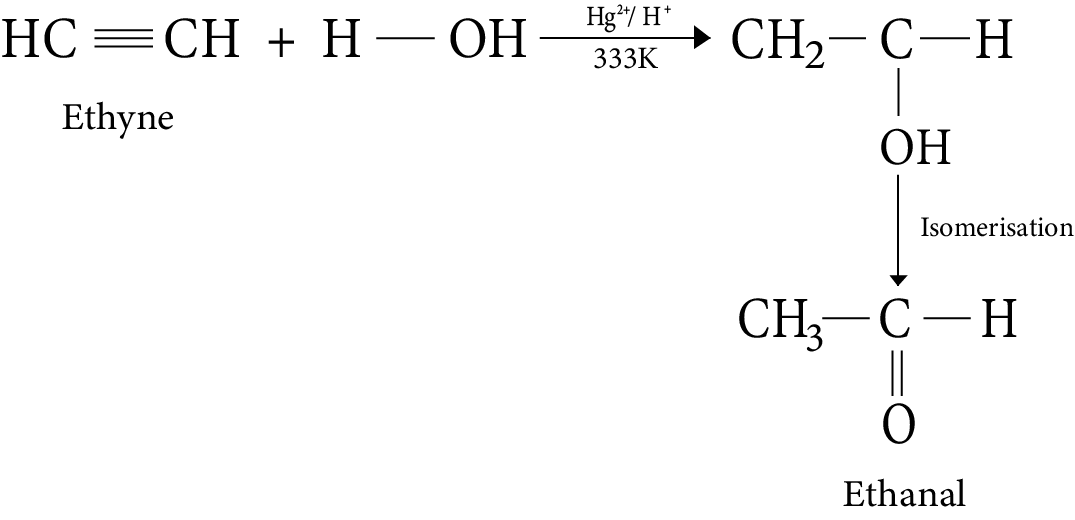
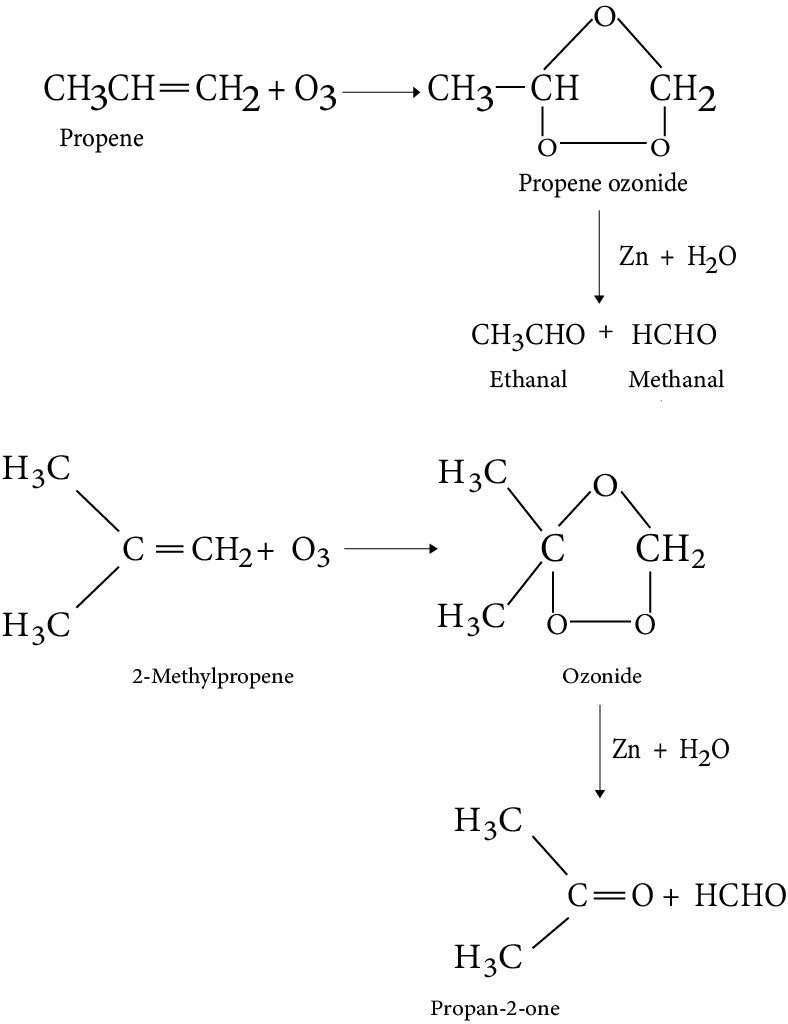
9. Ozonolysis
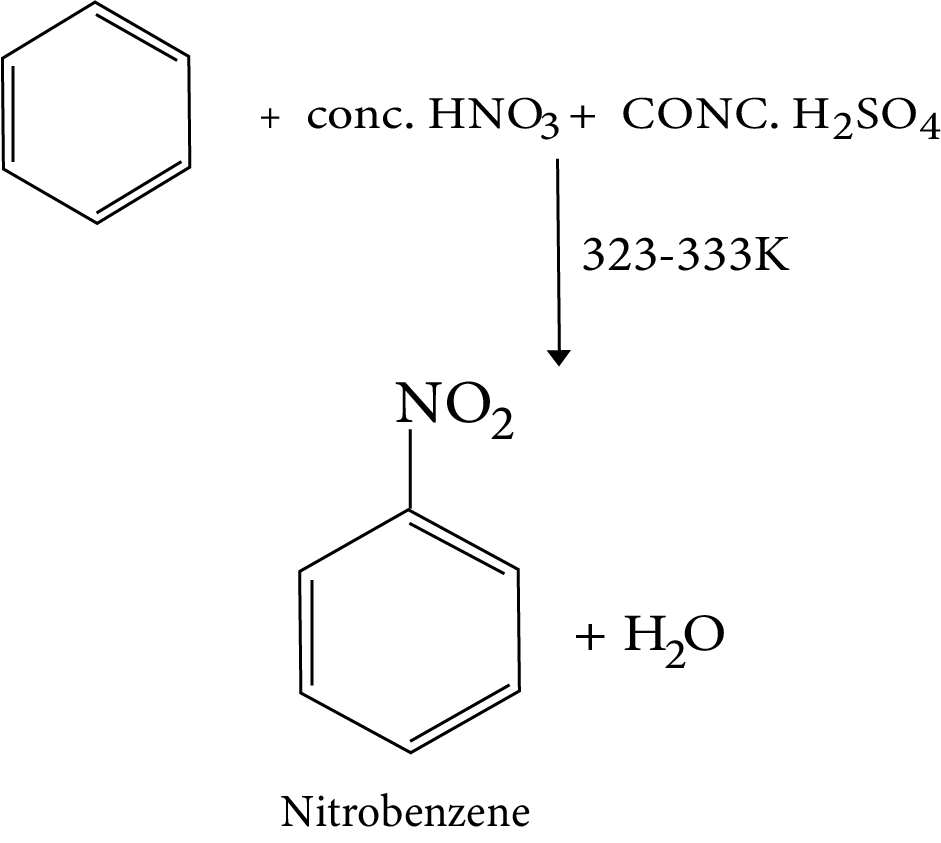
10. Nitration of Benzene
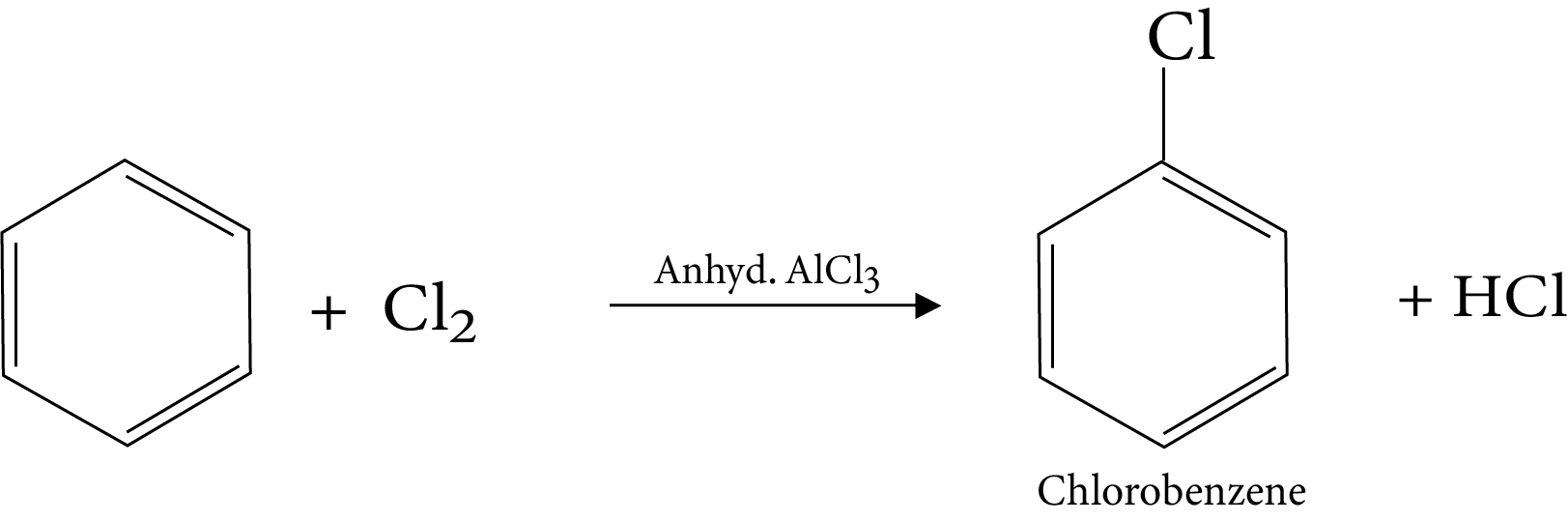
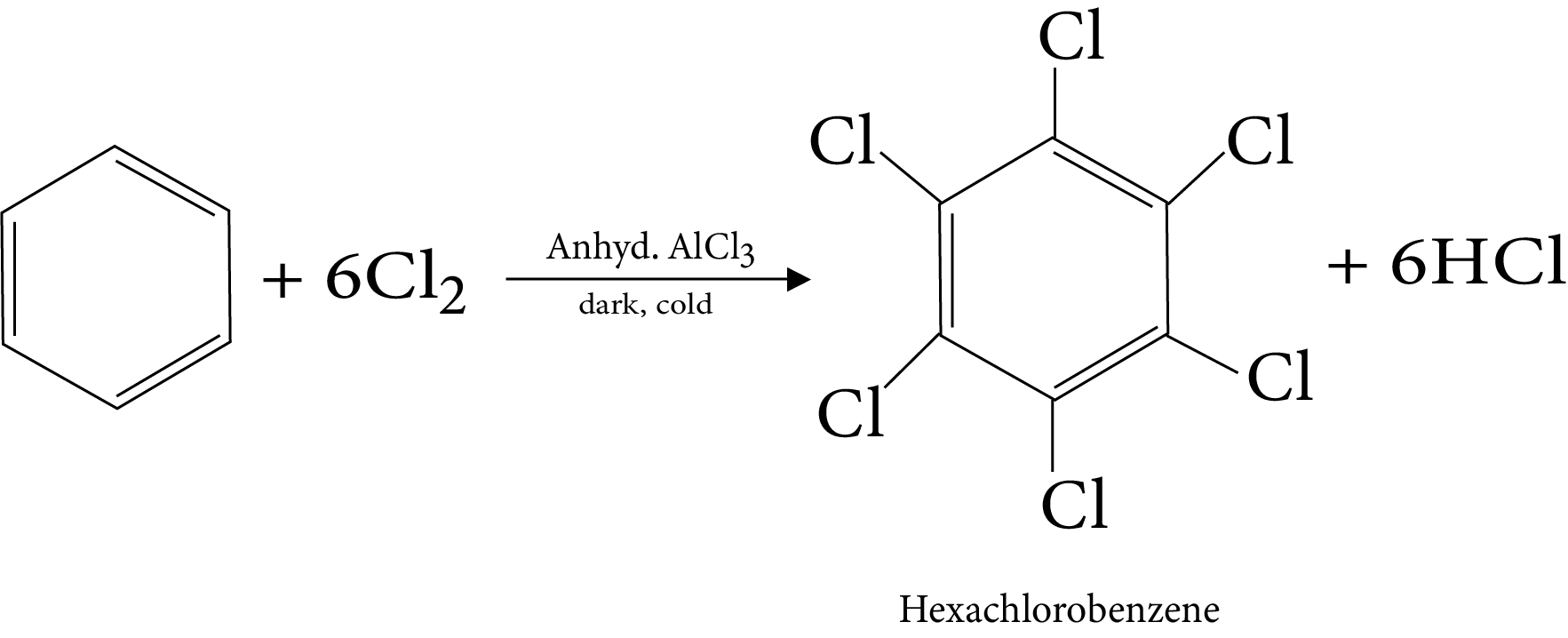
11. Halogenation
12. Sulphonation Reaction
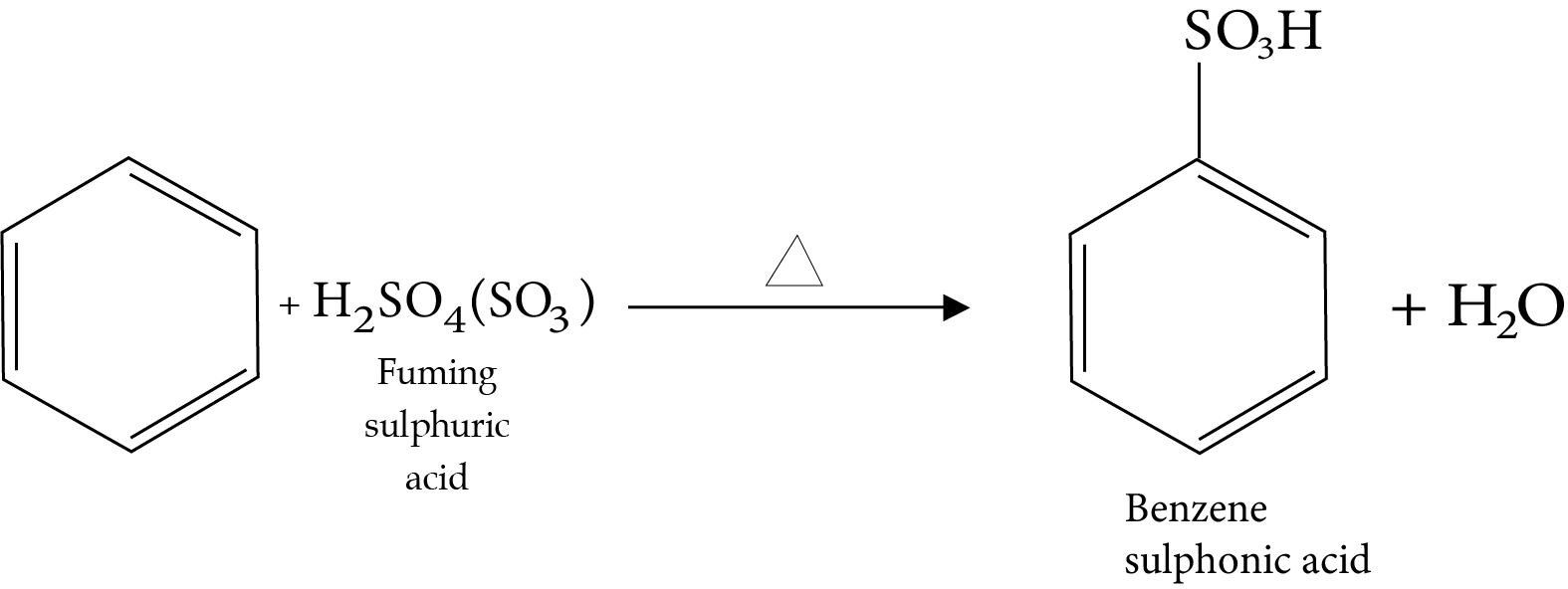
13. Friedel-Crafts Alkylation Reaction
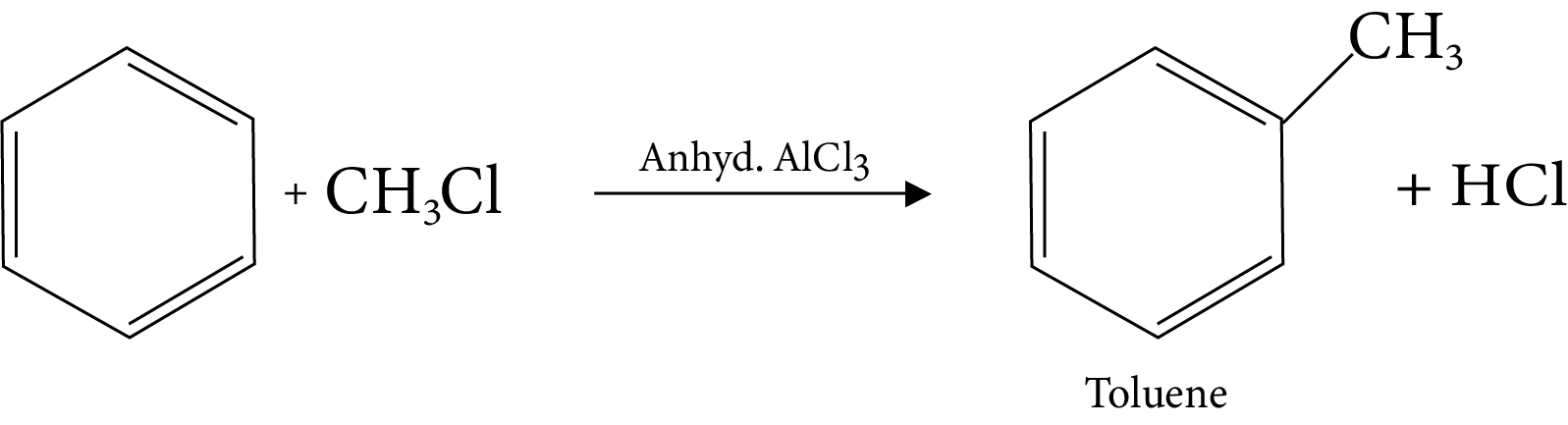
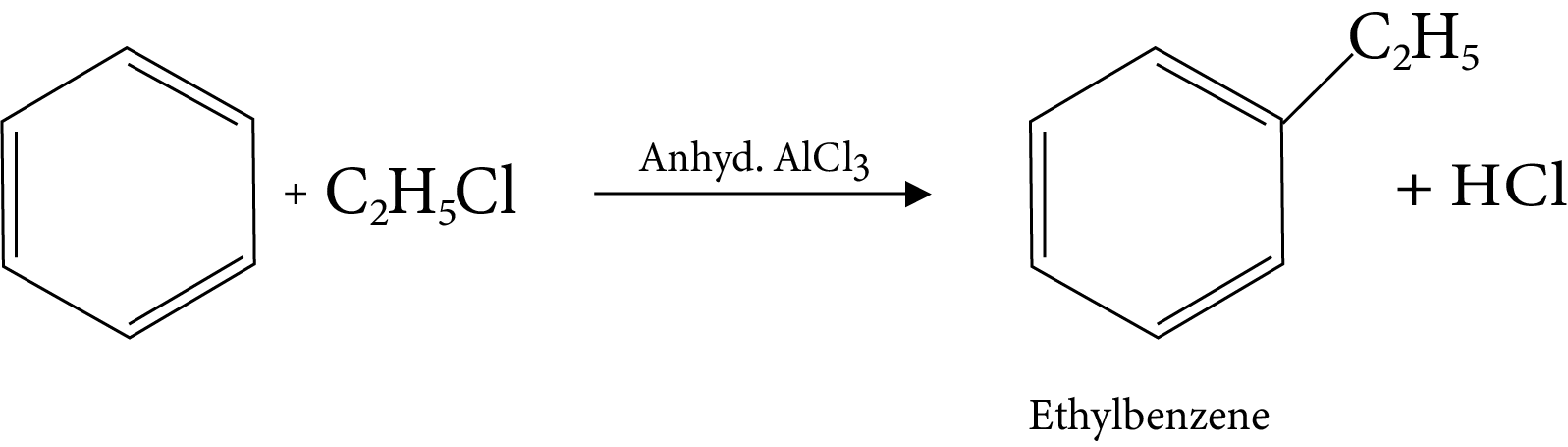
14. Friedel-Crafts Acylation Reaction
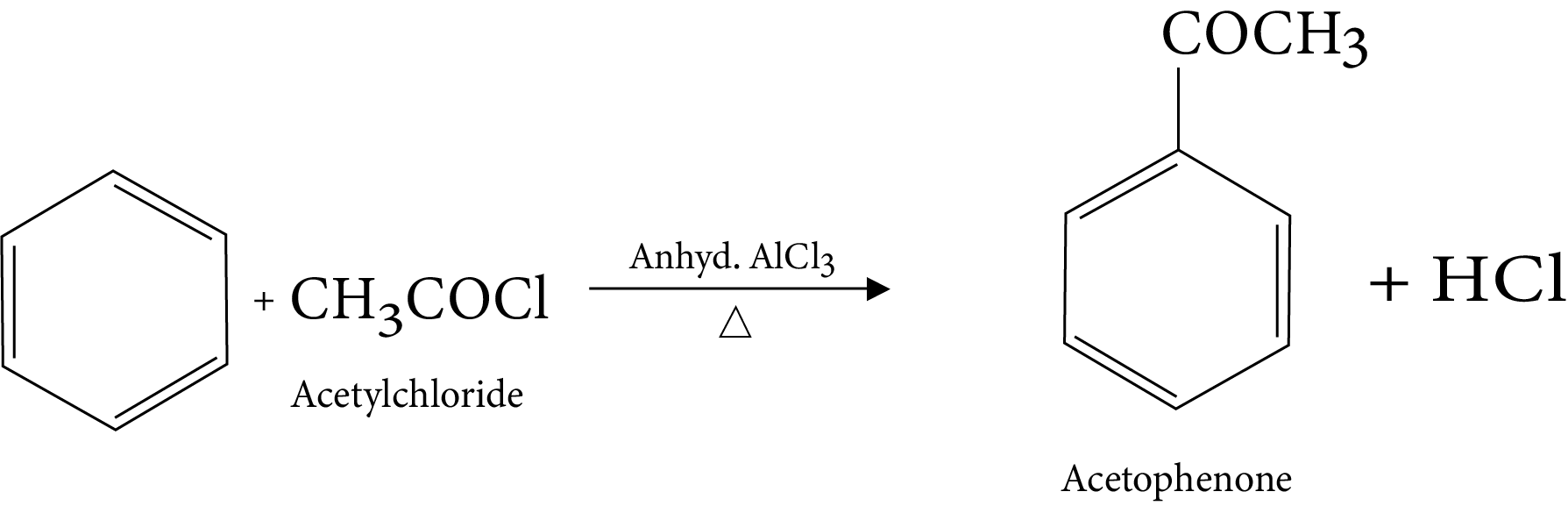
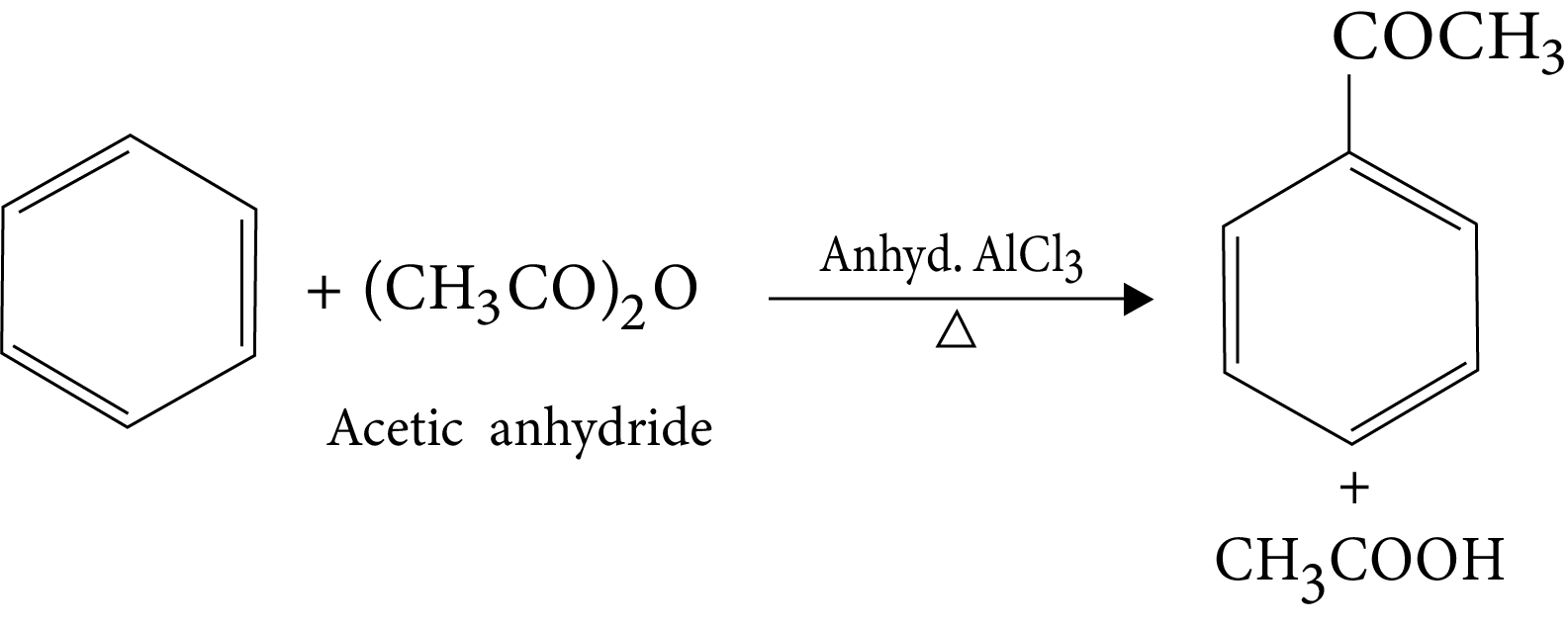
15. Reaction in excess of lewis acid
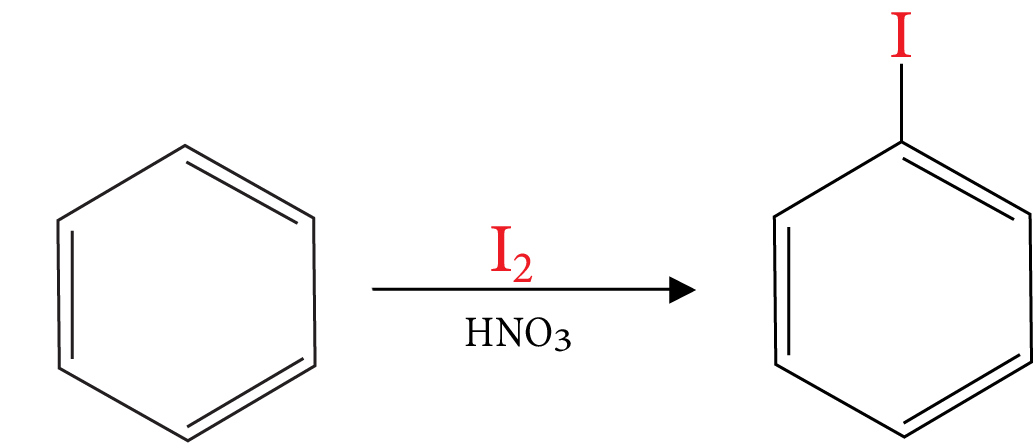
16. Iodination of Benzene
17. Hydrogenation
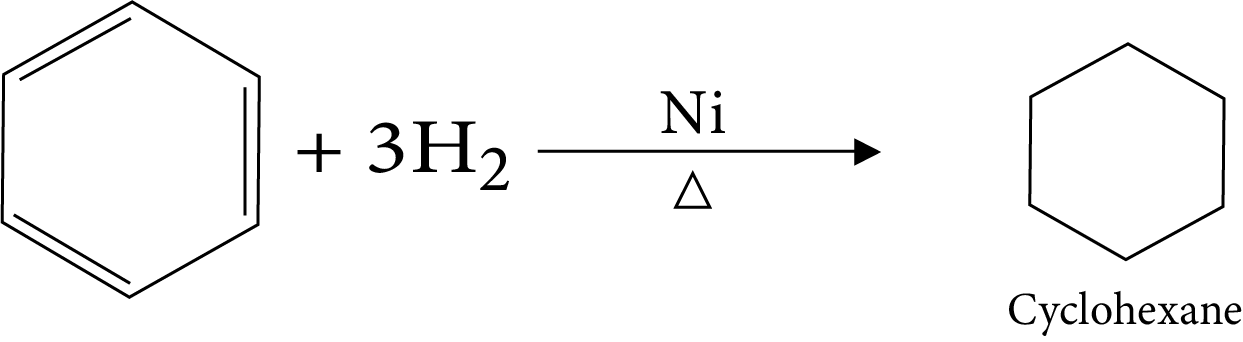
18. Halogenation
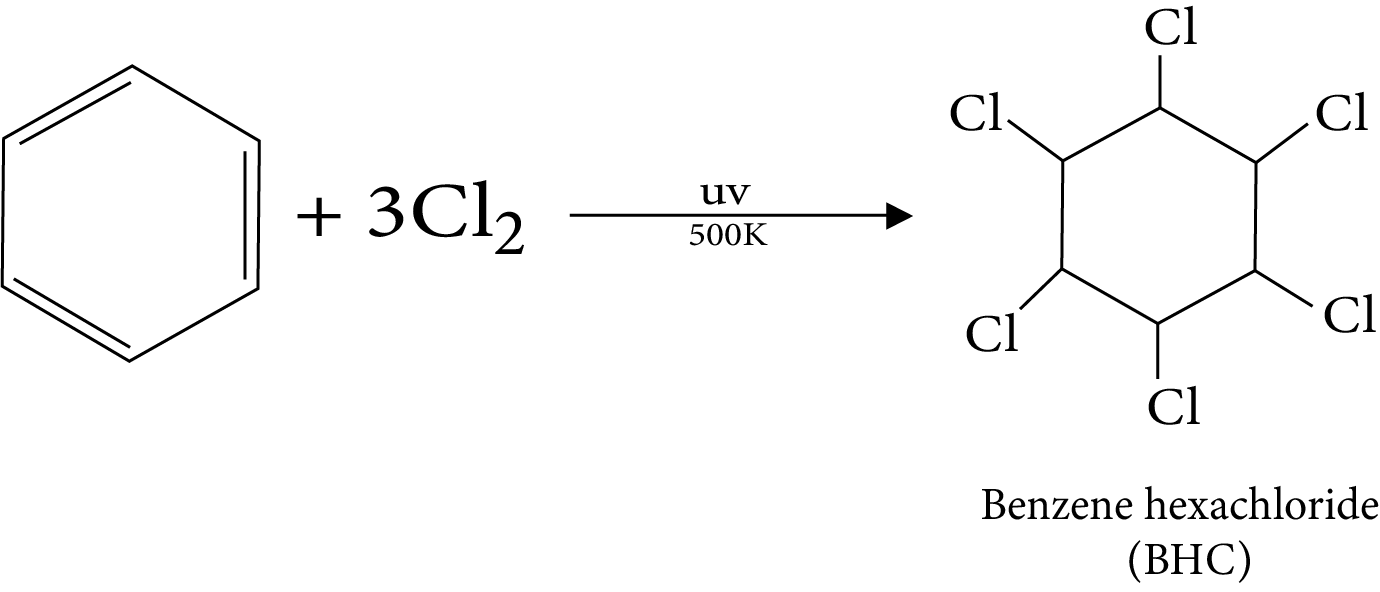
Uses of Hydrocarbon
Propane is a fuel that is also used to create chemicals. It is used in households for space and water heating, cooking, drying clothing, and fueling gas fireplaces, grills, and backup electrical generators.
Ethane is primarily used to manufacture ethylene, a feedstock for the production of plastics. Ethane is subsequently utilized by the petrochemical industry to produce a variety of intermediate products, the majority of which are turned into plastics. Ethane can also be utilized as a power production fuel, either alone or in combination with natural gas.
Normal butane and isobutane are mostly utilized as gasoline blending stocks. While some normal butane is used as a lighter fuel, the majority of it is blended into gasoline, particularly during the cooler months. Because demand for isobutane exceeds supply, isomerization is used to convert regular butane to isobutane.
Natural gasoline (also known as pentanes plus) is used in fuels and oil transportation. It can be blended into the fuels used in internal combustion engines, particularly motor gasoline.
Solved examples/problems from chapter
1. The addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give the mechanism.
Ans: While in the presence of benzoyl peroxide, the same reaction yields 1 - bromopropane. This is in accordance with anti-Makovnikov's rule. This happens in presence of peroxide and with HBr only. The reaction proceeds through free radical mechanism.
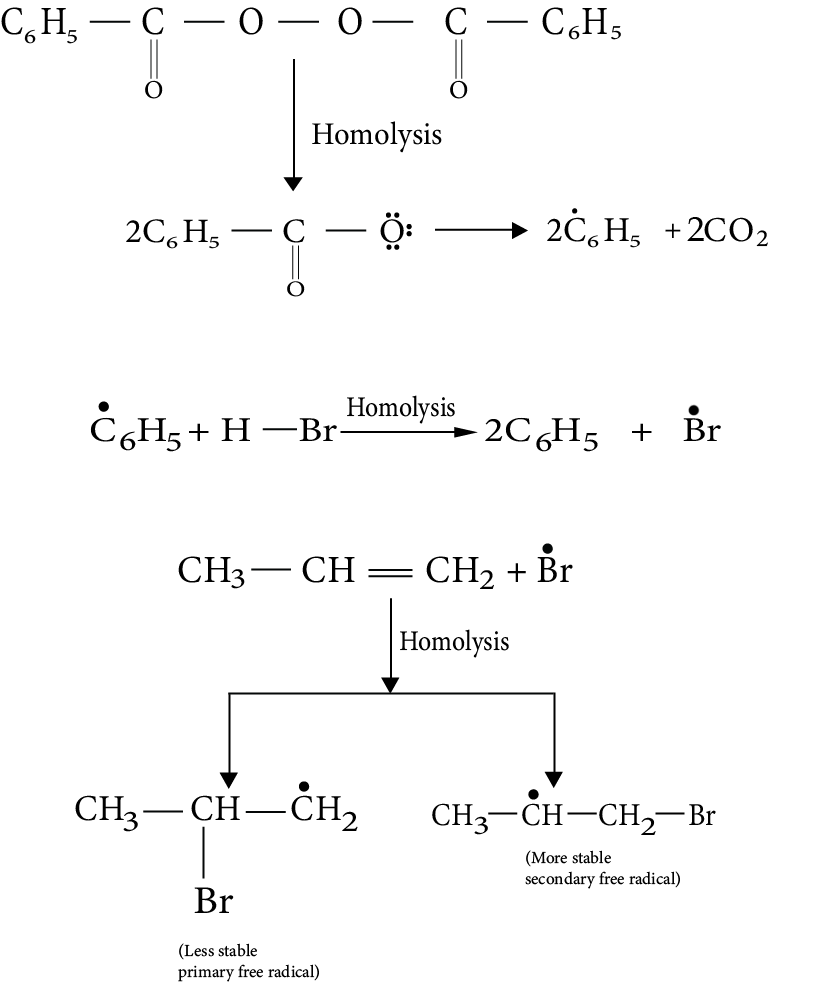
Key Point: The reaction involves the formation of primary and secondary free radicals. Secondary free radicals are more stable and preferentially formed.
2. Why is the Wurtz reaction not preferred for the preparation of alkanes containing an odd number of carbon atoms? Illustrate your answer by taking one example.
Ans: Wurtz reaction not preferred for the preparation of alkanes containing an odd number of carbon atoms because if we take two dissimilar alkyl halide as a reactant, the product will be a mixture of alkane but the reaction is by a free radical mechanism it will produce an alkene also.
Key Point: The mixture of alkanes are difficult to separate which makes the process unuseful.
Solved problems of Previous Year Question from chapter
1. The major product of the following chemical reaction is :
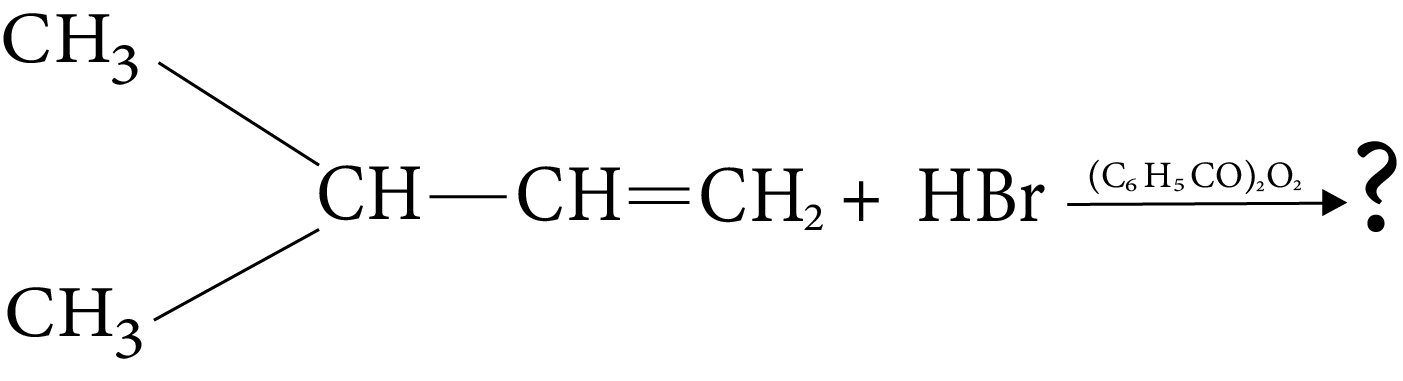
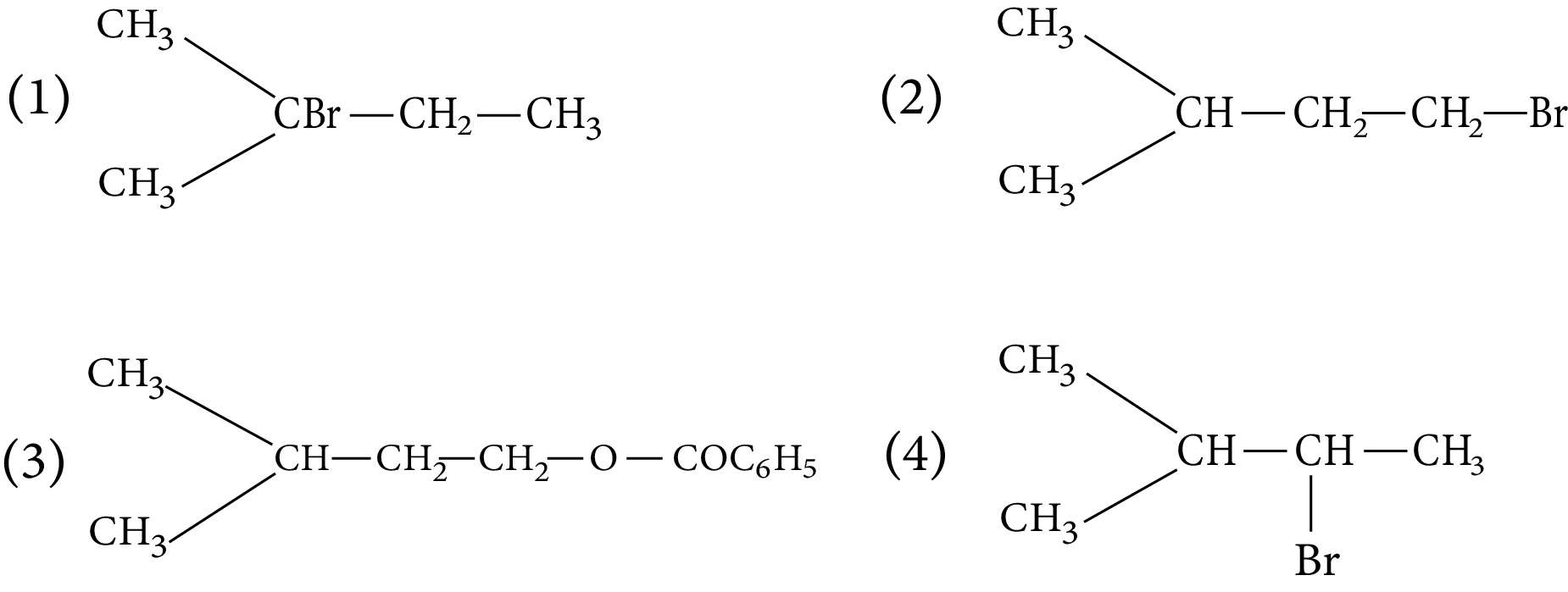
Ans: The correct answer to this question is the second option.
Trick: According to the anti-rule, Markovnikov's the principal product is peroxide (based on the stability of free radical intermediate)
2. The correct structure of 2,6-Dimethyl-dec-4-ene is :

Ans: The correct answer for this question is option 1.
Trick: Select the longest carbon chain and the priority of giving a lower number should be to double bond. Give numbers accordingly. Also, check the lowest sum rule.
3. The major product formed in dehydrohalogenation reactions of 2-Bromo pentane is Pent-2-ene.
This product formation is based on ?
(1) Huckel's Rule
(2) Saytzeff's Rule
(3) Hund's Rule
(4) Hofmann Rule
Ans: The correct answer for this question is the second option. This reaction follows the carbocation formation mechanism. More alkylated carbocation is more stable then less alkylated carbocation.
Trick: According to the saytzeff’s rule the more alkylated alkene will be formed from the elimination reaction.
Practice Questions:
1. How would you convert the benzene to cyclohexane
(Ans: Hydrogenation)
2. Out of benzene, m–dinitrobenzene, and toluene which will undergo nitration most easily, and why?
(Ans: Toluene)
Conclusion
In this hydrocarbon class notes we have provided important information regarding the chapter preparation properties and reactions of hydrocarbons such as important concepts, properties, reactions, etc.. Students should work on more solved examples and previous year's question papers for securing good grades in the NEET exams.
NEET Chapter - Hydrocarbons

 Share
ShareFAQs on NEET Chapter - Hydrocarbons
1. What are the most important hydrocarbon topics?
The important topics of hydrocarbon are:
Unsaturated hydrocarbons, alkyl halides, and carboxylic acid are used to make alkanes.
Alkanes' physical characteristics.
Alkanes have chemical characteristics.
Alkenes are made by combining alkynes, alkyl halides, vicinal dihalides, and alcohol.
Alkenes have physical properties.
2. What are the three different kinds of hydrocarbons?
Alkanes, alkenes, and alkynes are the three types of alkanes. Alkanes have just single bonds, alkenes have a double carbon-carbon link, and alkynes have a triple carbon-carbon bond. Aromatic hydrocarbons are a family of related chemicals formed by the chemical breakdown of certain pleasant-smelling plant extracts.
3. Why do hydrocarbons catch fire?
Hydrocarbons are flammable because the carbon in their structure is in its most reduced state, hydrocarbons are combustible.




















 Watch Video
Watch Video


