Prepare Thoroughly with Class 12 Chapter 16 Environmental Issues Notes for NEET
Students preparing for the NEET exam can now use the Environmental Issues Class 12 notes which cover important issues of lack of environmental awareness. People globally are confronted with a slew of new and difficult environmental issues. Pollution, ozone depletion, greenhouse effect, and deforestation are only a few of them. Students using the Environmental Issues NEET notes can learn more about these issues and understand the chapter well to prepare for the NEET.
These revision Environmental Issues class 12 NEET notes will help you prepare for this chapter with ease. This lengthy chapter is given in a condensed version helping in last-minute revision. Give your NEET exam preparation the right direction and learn about different environmental issues and how to overcome them using Environmental Issues Class 12 notes.
Access NEET Revision Notes Biology Environmental Issues
Introduction:
Pollution is defined as any change in the physical, chemical, or biological aspects of the environment that endangers human life.
Contamination is the presence of disease-causing organisms or their poisons.
There are two sorts of pollution: natural and man-made.
Natural sources pollute the environment. Volcanic eruptions, gas emissions such as NOx and ${O_3}$, UV radiation, cosmic rays, and so on.
Human activities generate anthropogenic contamination, often known as man-made pollution. For example, fuel combustion, deforestation, pesticides, and fertilisers.
Any substance or chemical that causes pollution is referred to as a pollutant.
Pollutants are Classified Into Two Groups Based on How They Establish Themselves.
Primary pollutants: These are present in the same state as when they are manufactured. Carbon monoxide and DDT are some examples.
Secondary pollutants, such as PAN, Ozone, $\mathrm{HNO}_{3}, \mathrm{H}_{2} \mathrm{SO}_{4}$ and others, are generated when main or primary pollutants react with each other in the presence of sunlight.
Peroxyacetyl nitrates (PAN) and ozone are produced when nitrogen oxides and hydrocarbons combine photochemically.
Secondary pollutants are more hazardous than primary pollutants.
Synergism is the term used for this phenomenon.
Pollutants are Classified into Two Groups Based on How Quickly They Degrade:
Pollutants that are decomposed or degraded by biological or microbiological action, such as domestic sewage, clothing, and paper, are examples of biodegradable pollutants.
Pollutants that are not digested or destroyed by living organisms or microorganisms are referred to as non-biodegradable pollutants.
- DDT, glass, plastics, aluminium cans, phenolic chemicals, pesticides, radioactive substances, and heavy metals such as mercury, lead, and cadmium are just a few examples.
Pollution is Classified Into the Following Types Based on the Amount of Pollutants Released:
Pollution from a single point source is referred to as a point source. For example, a chimney or a public sewer.
Line source pollution, for example, is pollution caused by automotive exhaust on roads.
Mining and industrial regions, for example, are both sources of air pollution.
- Water pollution from a diffuse source occurs over a vast area. Pesticides and fertilisers, for example.
According to Environmental Studies, Pollution Can Be Classified Into the Following Categories:
Pollution of the air or Air pollution
Pollution of the water supply or water pollution
Pollution of the soil or soil pollution
Pollution due to noise or Noise pollution
Pollution from radioactive sources or Radioactive pollution
Air Pollution:
Air pollution is defined as the presence or addition of foreign particles, gases, or contaminants in the air that have a negative impact on humans, animals, vegetation, and other living things.
Gases and particulates are the two main types of air contaminants.
Various gases and vapours of volatile substances, as well as compounds with a boiling point below $200^{\circ} \mathrm{C}$, are considered gaseous materials.
Particulate matter is made up of solid particles or liquid droplets (aerosols) that are small enough to stay suspended in the air, such as soot, smoke, dust, asbestos, fibres, pesticides, certain metals (such as Hg, Pb, Cu, and Fe), and biological agents such as dust mites and flower pollen.
Particles with a diameter of more than 10 μm settle out in the air in less than a day, however, particles with a diameter of 1 μm or less might stay suspended in the air for weeks.
- Human respiratory illnesses such as asthma and chronic bronchitis are caused and aggravated by suspended particulate matter in the lower atmosphere (troposphere).
The Effects of Major Air Pollutants
CO (Carbon Monoxide) :
It is the major air pollutant (or the most toxic gas) generated by car smoking.
Carbon monoxide is an extremely poisonous gas that interacts with haemoglobin in the blood and prevents oxygen from being transported.
When breathed in significant concentrations, it affects respiration and causes death due to asphyxiation.
Hydrocarbons (3,4 Benzopyrene, CH4, Benzene) :
These are primarily released by automobiles and the combustion of fossil fuels (coal, petrol, diesel).
The most abundant hydrocarbon in the atmosphere is methane CH4, which is primarily produced in marshy areas and rice fields.
Lung cancer is caused by hydrocarbons.
Ethylene:
Falling leaves for no apparent reason, and flowering buds that fall before their time.
Oxides of Nitrogen ($NO, N{O_2}$):
In automobiles, fossil fuels are burned.
It contributes around 10% of all pollutants.
These nitrogen oxides release ozone and generate photochemical haze in the atmosphere.
Acid rain is also caused by nitrogen oxide. Inhaling nitrogen oxides and ozone causes respiratory disorders in humans, including emphysema, bronchitis, lung edema, and lung cancer.
Sulphur Oxide ($S{O_2}, S{O_3}$):
Coal combustion, smelters, and oil refineries are the principal sources of sulphur oxides, which are the most damaging gaseous pollutants. It accounts for around 18% of all air pollutants.
$S{O_2}$ contamination is detected by lichens and mosses. Sulphur oxides in the atmosphere cause acid rain and smog.
- $S{O_2}, S{O_3}, N{O_2}, NO, CO, C{O_2}$ are examples.
Air Pollution Control:
Arresters and Scrubbers are two devices that are used to remove particle air pollution.
Particulate matter is separated from contaminated air using arresters. Arresters come in a variety of shapes and sizes.
Cyclonic Separators and Trajectory: These are typically used to separate certain substances from industrial pollutants with the least amount of moisture. These separators function on the principle of centrifugal force to separate dust.
The most efficient technology for removing fine particulate pollution is the electrostatic precipitator.
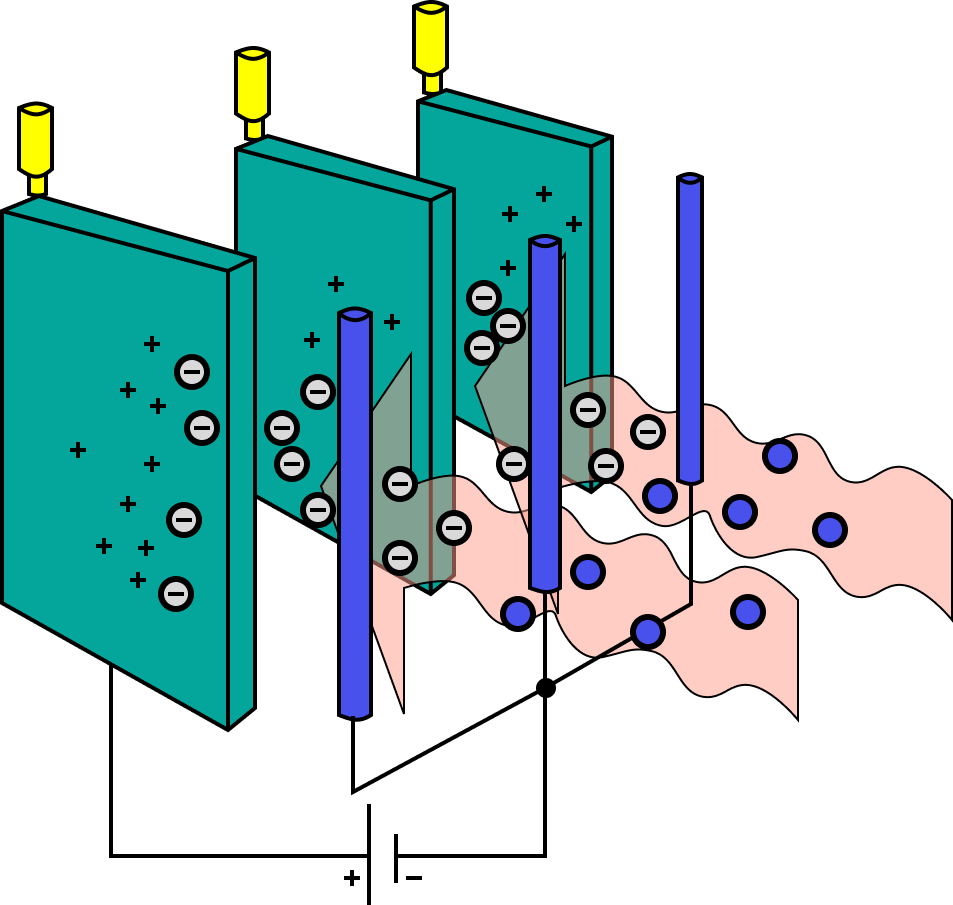
Electrostatic Precipitator
Electrostatic precipitation devices work electrically.
charging dust particles and collecting them on a platform with a different charge.

Fumes Liberating From Factories
Scrubbers are used to remove dust and gases from the air. The two types of scrubbers utilised for dust separation are wet and dry.
Gaseous Pollutant Control
Gaseous pollutants are controlled via combustion, absorption, and adsorption procedures.
Combustion is the process of totally burning oxidizable gaseous contaminants at a high temperature. Combustion control of gaseous pollutants is used in the petrochemical, fertiliser, paint, and varnish industries.
Gaseous contaminants are absorbed in suitable absorbent materials using this technology.
Adsorption: This approach is used to regulate poisonous gases, vapours, and combustible substances that cannot be removed or transferred efficiently using other methods. Pollutants in the air are adsorbent on huge solid surfaces.
Other Points to Remember:
Engines should not be kept running when cars are at rest, among other methods.
The smoke is reduced when a barium compound is combined with gasoline.
It is also critical to monitor the quality of gases emitted by companies.
It is not advisable to build industries in a single location.
After filtering and purification, the smoke should be discharged into the atmosphere (by cyclone collector or electrostatic precipitators).
Effect of Pollutants on the Air
Smog, acid rain, the greenhouse effect (global warming), and ozone layer depletion are four important environmental consequences caused by air pollution.
Smog
It is made up of a mixture of smoke and fog.
In plants, it produces silvering/glazing and necrosis, whereas, in humans, it causes allergies and asthma/bronchitis.
There are two forms of smog.
Classic or London Smog, or Sulphurous Smog - It is caused by the presence of ${H_2}S, S{O_2}$, smoke, and dust particles at low temperatures. Secondary Pollutants are not present in it.
In 1905, it was first noticed in London during the winter months. It is formed by the combustion of coal in both home and industrial settings.
Photochemical Smog, sometimes known as Los Angel's smog, was first noticed in the middle of the day in 1943 in Los Angeles. Smog was assumed to be caused by the combustion of petroleum in automobiles.
At high temperatures, photochemical smog forms over cities and towns. The photochemical smog is generated when two air pollutants, nitrogen oxides (primarily $N{O_2}$) and hydrocarbons (HC), combine in the presence of UV rays from sunlight to produce ozone (${O_3}$) and PAN (peroxy acetyl nitrate).
Breathing ozone causes headaches, respiratory distress, and weariness in the respiratory and nervous systems.
It also irritates the eyes and aggravates asthma. Potato, Alfalfa, and spinach crops have been observed to be 50% destroyed by ozone.
It also harms tobacco, tomato, and pine leaves, as well as grapefruits. In addition, the PAN inhibits Hill's photosynthetic reaction.
Acid Rain:
Acid rain is a type of precipitation that contains dangerous levels of nitric and sulphuric acids, which are created principally by nitrogen and sulphur oxides that are released into the atmosphere.
Oxygen is released into the air by power plants burning coal and oil, and Nitrogen dioxide is released into the air by the automotive exhaust.
The pH of pure rain is around 5.6, while the pH of acid rain is below 5.6.
Acid rain reduces trees' ability to withstand cold temperatures, and weakened trees are killed by the cold and become more susceptible to disease.
Acid rain dissolves lead, mercury, and calcium in soils and rocks, releasing them into rivers and lakes. Metals may accumulate in fish and subsequently be passed on to humans through the food chain.
Steel, paint, plastics, cement, and marble are among the materials that are harmed by acid rain.
Global Warming and the Greenhouse Effect
Carbon dioxide is not usually thought of as a pollutant, but at greater concentrations, it forms a thick layer above the earth's surface that prevents heat from radiating from the surface.
The temperature of the earth's surface rises as a result of this, which is known as the "Greenhouse effect" or global warming.
CO2, Methane, Nitrogen, Oxygen and CFC are the most common greenhouse gases.
The presence of a CO2 layer encircling the world allows short wavelength incoming solar radiation to pass through, but prevents long wavelengths of outgoing heat radiation from the earth's warm surface, keeping the planet warm.
Global warming is the result of this increase in the global mean temperature.
Consequences of Global warming:
Effect on Weather and Climate: By 2100, the average temperature is expected to rise by 1.4 to 5.8 degrees Celsius. The capacity of the atmosphere to hold moisture rises as it warms. Changes in precipitation patterns are caused by all of these factors. Human health is being harmed as a result of climate change.
Global warming is to blame for rising sea levels as well as the melting of glaciers and ice sheets in Greenland.
Effects on Species: Many species are projected to migrate poleward or too high elevations in mountain regions as a result of rising global temperatures
Depletion of Ozone
The amount of ozone in the atmosphere is decreasing. However, the concentration of ozone in the stratosphere is highest at a height of 16 km to 25 km on Earth.
The ozone layer is 3mm thick at normal temperature and pressure. (However, the ozone layer thickness at the poles is 4mm.)
Because the ozone layer is depleted, dangerous UV radiation reaches the planet, causing skin cancer and acting as potent mutagens.
The ozone layer is depleted or reduced by aerosols such as chlorofluorocarbons, which are emitted into the atmosphere by refrigerators, air conditioners, and jet planes. This is known as ozone depletion, and the compounds involved are known as O.D.S. (ozone-depleting substances).
The ozone layer is depleted by a variety of contaminants, including CFCs (15 percent of overall depletion), nitrogen oxide (3.5 percent), and halogens (chlorine).
CFCs have the highest ODP (ozone depletion potential) due to chlorine emission.
Noise Pollution:
Noise pollution is defined as unwanted sound that exceeds 80 decibels (dB).
The decibel level of noise pollution is measured in dB.
Noise pollution can harm the heart, elevate blood cholesterol, and possibly raise blood pressure, among other things.
Noise pollution can be reduced by reducing noise levels at the source, protecting people from noise, and monitoring noise pollution.
Water Pollution:
Water pollution is defined as the introduction of substances (organic, inorganic, biological, or radioactive) or factors (e.g., heat) that deteriorate the quality of water to the point where it is either a health danger or unfit for use.
Physical, chemical, or biological pollution can all occur in water.
Changes in the physical qualities of water, such as colour, taste, odour, temperature, and turbidity, are examples of physical pollution.
A change in the chemical characteristics of water causes chemical pollution. pH, dissolved oxygen, inorganic or organic compounds, heavy metals, and other factors are among them.
Fluorides, chlorides, phosphates, and nitrates are examples of inorganic chemicals, while phenols, colours, pesticides, and chloro compounds are examples of organic chemicals.
The presence of living organisms in water, such as algae, fungi, bacteria, and viruses, causes biological pollution.
Pollution Sources and Effects of Pollutants:
In India, especially in villages, water pollution is a severe health threat. It is estimated that 50-60% of the Indian population is affected by ailments brought on by it. It is thought to be responsible for 30-40% of all deaths.
Domestic sewage and industrial effluents are the main causes of water contamination and contaminants.
Sewage and Household Wastes
Sewage containing human faeces, urine, kitchen and textile cleaning, organic wastes, industrial wastes, and other contaminants is commonly discharged into bodies of water, causing pollution.
Villagers frequently bathe, wash their livestock, and wash their clothes in the same pond. Infectious agents for cholera, typhoid, dysentery, jaundice, and skin illnesses can be found in such water.
Because sewage supplies food for decomposers, their population grows.
Decomposers/microorganisms responsible for sewage decomposition use the majority of the oxygen dissolved in water. As a result, BOD (Biological oxygen demand or Biochemical oxygen demand) rises drastically in this water.
BOD is the quantity of oxygen in milligrams required by microorganisms to metabolise the waste in one litre of water at 20°C for five days.
Pure drinking water has a B.O.D. of less than 1ppm (mg/litre).
A weak organic waste has a BOD of less than 1500 mg/litre, a medium organic waste has a BOD of 1500–4000 mg/litre, and a strong organic waste has a BOD of more than 4000 mg/litre.
BOD is directly proportional to the degree of water contamination.
Chemical Oxygen Demand (C.O.D.) is the quantity of oxygen necessary to oxidise all polluting compounds in five days using one litre of water at 20°C. COD has a substantially higher value than BOD.
Phosphorus and nitrogen molecules are present in sewage and are required for algae growth. These build up in dirty water, resulting in an overabundance of algae on the water's surface. The term "water bloom" refers to an excessive amount of algae development.
Industrial Discharges
Wastewater is typically discharged into ponds, lakes, and rivers by industries.
Heavy metals (mercury, lead, copper, arsenic, and cadmium), inorganic pollutants (acids, alkalies, and bleaching liquors), and organic pollutants (mercury, lead, copper, arsenic, and cadmium) are all found in industrial effluent (phenol, naphtha, proteins, aromatic compounds, cellulose fibres)
On land and in the sea, industrial effluents are the most dangerous pollutants.
Mercury is a metal that is found in Coal combustion, smelting of metallic ores, and the paper and paint industries all emit it.
Mercury has a long half-life. It is converted to the water-soluble dimethyl form in water and enters the food chain (biomagnification).
It kills fish and poisons the rest of the wildlife. Humans that consume poisoned animals suffer a paralysing condition known as Minamata , which is marked by sensory impairment, diarrhoea, hemolysis, meningitis, and death.
Microorganisms do not digest non-biodegradable pollutants such as Al, Hg, Fe, DDT, insecticides, and the phenolic compound ABS (Alkyl benzene sulphonate).
Biological amplification is the process of these substances accumulating in tissue at increasing concentrations as they move up the food chain. The top consumer has the largest concentration.
DDT was banned for agricultural usage in India in 1985. It demonstrates biomagnification.
Lead is a contaminant that can be found in the air, soil, and water.
Smelters, battery manufacturing, paint, chemicals, and pesticide manufacturing, as well as automotive exhaust, are all causes of lead pollution.
Lead is a persistent contaminant that has the potential to biomagnify.
It causes anaemia, headaches, vomiting, colic, muscle weakness, bluish lines around the gums, loss of appetite, and liver, kidney, and brain damage.
Metal industries, welding and electroplating, insecticides, and the phosphate industry all release cadmium (Cd) into the environment.
Cd accumulates in the kidneys, liver, pancreas, and spleen due to biological amplification.
It causes itai-itai illness (joint disease), hypertension, anaemia, diarrhoea, and liver and kidney damage.
Oil:
Some oil spills into the water's surface during the extraction and transportation of oil from the sea to various locations. Refineries also dump a lot of oil into rivers as part of their effluents.
Spreading oil on the water's surface limits oxygenation and reduces aquatic plants' photosynthetic activities. Animal life is being wiped out due to a lack of oxygen, food, and the poisonous effects of oil.
- Oil spilled on the water's surface might catch fire, killing all biological life.
Thermal Pollution:
Many industries, power generation plants, and thermal power plants produce hot water.
The addition of hot water effluents to water bodies causes thermal pollution, which results in a rise in water temperature.
Warmer water has less oxygen in it. As a result, the rate of breakdown of organic materials slows down.
Green algae are replaced with less desirable blue-green algae in hot water.
Many creatures, such as salmon and trout, cannot reproduce in hot water.
Pollution with Radioactive Elements
The property of the abrupt emission of various charged particles and radiation (rays) by the decay of atomic nuclei is known as radioactivity, and the elements are known as radioactive elements.
Radioactivity contributes to air, water, and soil pollution, as well as being exceedingly destructive to creatures.
The following are the various sources of radioactive materials:
Natural resources include: Radium 224, Uranium 235, Uranium 238, Thorium 232, Radon 222, Potassium 40, and Carbon 14 are examples of cosmic rays.
Man-made radiation is discharged into the atmosphere during the mining and purification of thorium and plutonium, as well as the manufacturing of nuclear weapons.
Pollution of the Soil (Solid Waste)
Soil pollution is the unfavourable change of soil by the addition or removal of chemicals and variables that reduce soil productivity, plant product quality, and groundwater.
Pesticides, fertilisers, industrial wastes, salts, tin, iron, lead, copper, mercury, aluminium, plastics, paper, glass, broken bottles, and abandoned food are all examples of soil contaminants.
Insecticides, fungicides, algicides, weedicides or herbicides, rodenticides, and other pesticides are examples.
Pesticides have a broad spectrum of effects, including on other animals, humans, and even plants. As a result, they're also known as biocides.
Chlorinated hydrocarbons such as DDT (dichloro diphenyl trichloroethane), BHC (benzene hexachloride or gammexane), aldrin, dieldrin, endrin, and heptachlor are used as pesticides.
Chlorinated hydrocarbons have a long half-life, are fat-soluble, and can biomagnify.
Chlorinated hydrocarbons such as DDT (dichloro diphenyl trichloroethane), BHC (benzene hexachloride), aldrin, and dieldrin are insecticides.
DDT and other chlorinated hydrocarbons impact the central nervous system, causing softness of the brain, liver cirrhosis (liver cancer), cerebral hemorrhage, cancer, hypertension, thinning of egg shells in birds, sex hormone abnormality, and gonad development failure.
- Weedicides (also known as herbicides) are metabolic inhibitors that interrupt photosynthesis and other metabolic functions in plants, killing them.
Points to Remember
Major issues relating to environmental pollution and depletion of valuable natural resources vary in dimension from local, regional to global levels.
Main cause of air pollution is burning of fossil fuel in industries and automobiles.
The most common source of pollution of water bodies is domestic sewage which reduces dissolved oxygen but increases BOD.
Domestic sewage is rich in $\mathrm{N}_{2}$ and phosphorus which causes eutrophication and algal blooms.
Industrial waste waters are often rich in toxic chemicals, especially heavy metals and organic compounds.
Municipal solid wastes are disposed of in landfills.
Disposal of hazardous wastes like defunct ships, radioactive wastes and e-wastes requires additional efforts.
Soil pollution primarily results from agricultural chemicals (e.g. pesticides) and leachates from solid wastes deposited over it.
Two major global environmental issues are the increasing greenhouse effect, which is warming-up Earth and ozone depletion in the stratosphere.
Enhanced greenhouse effect is mainly due to increased emission of carbon dioxide, methane, nitrous oxide and CFCs and also due to deforestation.
Ozone in the stratosphere, which protects us from harmful effects of ultraviolet radiation, is depleting fast due to emission of CFCs thus, increasing the risks of skin cancer, mutation and other disorders.
The degradation of natural resources can occur, not just by the action of pollutants but also by improper resource utilisation practices.
Deforestation is the conversion of forested areas to non-forested ones.
Importance of Class 12 Chapter 16 Environmental Issues Notes
This Biology chapter of Environmental Issues talks about different problems people are facing on a global level. From air pollution to water pollution, there are multiple things happening. In the Environmental Issues class 12 NEET notes, students will have a complete picture on how air pollution is creating a hazardous life for people. Pollutants limit agricultural growth and yield while also causing plants to die prematurely. Pollution's negative impact on all living organisms is determined by-
Pollution concentration
The length of the exposure
Organisms involved
Students using these Environmental Issues NEET notes will further learn about water pollution and its controlling methods. It gives a clear picture of how water bodies are the major lifeline of human beings and animals. Prevention of water pollution is an essential part. Students will learn about integrated waste water treatment. Ecological sanitation is a long-term solution for disposing of human waste that uses dry composting toilets. This is a practical, sanitary, and cost-effective method of disposing of human waste.
Then there are radioactive wastes. The radiation generated by nuclear wastes is particularly harmful to biological beings because it generates a high rate of mutation. After proper pre-treatment, radioactive waste should be stored in suitably insulated containers and buried 500 metres beneath the earth's surface.
Benefits of Vedantu's Environmental Issues Class 12 NEET PDF
Subject matter experts of Vedantu have created these revising notes in an easy tone that makes students understand. It will assist pupils in grasping new concepts more quickly and effectively. All definitions, problems, and solutions are mentioned in a good way. All of the terms used in the formulas will be thoroughly explained.
The revision notes' condensed structure will allow you to study and prepare this chapter in no time. Students can use Environmental Issue notes NEET PDF download to revise before the exam. The best part is students will be able to understand the concept well and appear for the NEET exam.
Every chapter and concept is presented in a simple language. Students who are in their 12th standard can practice it without much difficulty.
The ultimate aim is to score high marks in NEET exam using Environmental Issues Class 12 NEET PDF.
Download Environmental Issues Class 12 Notes PDF
Complete your NEET preparation with the use of Environmental Issues Class 12 notes PDF. These notes provide a straightforward explanation that will help you learn about the chapter in a better way. Find out different types of environmental issues the world is facing and how one can control the same. To clear up any concerns and improve your NEET scores, get the notes available for free download.
FAQs on NEET Revision Notes for Class 12 Biology Chapter 16 Environmental Issues
1. What is organic farming?
Organic farming is a zero-waste method that involves waste products from one process being cycled in as nutrients for other processes to maximise resource use and improve production efficiency. Dairy management, composting, water harvesting, and agriculture are all part of a chain of activities that support each other and allow for a very cost-effective and long-term endeavour.
2. What are solid wastes?
Municipal solid wastes are wastes collected and disposed of by the municipality from homes, offices, and hospitals, among other places. Paper, plastics, food waste, metals, leather, textiles, and other materials fall under these wastes. Although burning minimises the volume of waste, open dumps are frequently used as a breeding ground for rats and flies.
3. What is Green House Effect?
The greenhouse effect is a naturally occurring issue causing the earth's surface to warm when carbon dioxide and methane gas concentrations rise.
4. Are UV Rays very harmful?
Yes, UV rays are very harmful to human beings and other living creatures in all senses. In recent years, there is a rise in UV rays. These UV rays are quite strong and can lead to some health issues, such as cancer and other skin and eye diseases. Moreover, they carry more energy compared to UVA rays.

























