




Concepts of Chemistry States of Matter for NEET Chemistry
The characteristics of chemical systems that we are familiar with represent the bulk properties of matter, i.e. the qualities of matter that we are familiar with. Properties that apply to a vast number of things, such as atoms, ions, or molecules. The molecules in a liquid do not boil, but the bulk does. Wetting qualities exist in water molecules in a collection; molecules do not wet on their own. Water is a kind of ice. It can take the form of a solid, a liquid, or a gas. In a gaseous state, water vapour or steam. Ice, water, and steam have a wide range of qualities. In all three stages, the chemical composition of water remains the same.
Important Topics of Chemistry States of Matter Chapter
Intermolecular Forces
Thermal energy
The gaseous state
The Gas Laws
Ideal gas equation
Kinetic energy and molecular speeds
Kinetic molecular theory of gases
Behaviour of real gases
Liquefaction of gases
Liquid state
1. Intermolecular Forces
The forces of attraction and repulsion between interacting particles are known as intermolecular forces (atoms and molecules). This phrase is used to describe does not take into account the electrostatic forces that exist between the two ions with opposing charges and the forces that hold a molecule's atoms together.
(a) Dispersion Forces or London Forces
Because their electronic charge cloud is evenly distributed, atoms and nonpolar molecules are electrically symmetrical and have no dipole moment. Even with such atoms and molecules, however, a dipole can form for a brief period of time.
The interaction energy is inversely proportional to the sixth power of the distance between two interacting particles, and these forces are always attractive.
(b) Dipole-Dipole Forces
Between molecules with permanent dipoles, dipole-dipole forces act. The dipoles' ends have "partial charges," which are represented by the Greek character delta .
Partial charges are always less than the unit electronic charge. The polar molecules interact with their neighbours.
(c) Dipole–Induced Dipole Forces
This form of attraction exists between polar molecules that have a persistent dipole and molecules that do not have a permanent dipole.
By deforming the electronic cloud of the electrically neutral molecule, the permanent dipole of the polar molecule produces a dipole on the electrically neutral molecule.
(d) Hydrogen Bond
This bond is found in compounds that include extremely polar N–H, O–H, or H–F bonds. Although hydrogen bonding is thought to be limited to the elements N, O, and F, species like Cl can also participate.
The energy of a hydrogen bond can range from 10 to 100 kJ mol–1. Because hydrogen bonds have such a large quantity of energy, they are a potent factor in influencing the structure and characteristics of many substances, such as proteins and nucleic acids.
The coulombic interaction between the lone-pair electrons of one molecule's electronegative atom and the hydrogen atom of another molecule determines the strength of the hydrogen bond.
2. Thermal Energy
Thermal energy is a body's energy derived from the motion of its atoms or molecules. It is directly proportional to the substance's temperature. It is the measure of the average kinetic energy of matter particles and is consequently responsible for particle movement. Thermal motion is the name given to this type of particle movement.
3. Intermolecular Forces vs Thermal Interactions
When molecular interactions are weak, molecules do not stick together to form a liquid or solid until the temperature is lowered to minimise thermal energy. Although molecules get very close to one another and intermolecular tensions are at their highest, gases do not liquefy solely due to compression.
When the thermal energy of molecules is reduced by lowering the temperature, the gases become more easier to liquefy.
4. The Gaseous State
We spend our entire lives immersed in the ocean of air, which is made up of a variety of gases. We spend our lives in the troposphere, the lowermost layer of the atmosphere that is held to the earth's surface by gravitational force.
The physical properties that define the gaseous state are as follows.
Gases can be compressed quite easily.
Gases apply pressure in all directions equally.
The density of gases is substantially lower than that of solids and liquids.
Gases do not have a definite volume or shape.
Gases mix uniformly and fully in all amounts without any mechanical assistance, assuming the container's capacity and shape.
5. The Gas Laws
(a) Boyle’s Law (Pressure-Volume Relationship)
Robert Boyle came to the conclusion that the pressure of a fixed amount (i.e., number of moles n) of gas varies inversely with its volume at constant temperature based on his experiments. Boyle's law is the name for this. It can be written as - P1/V1=P2/V2 at the constant temperature.
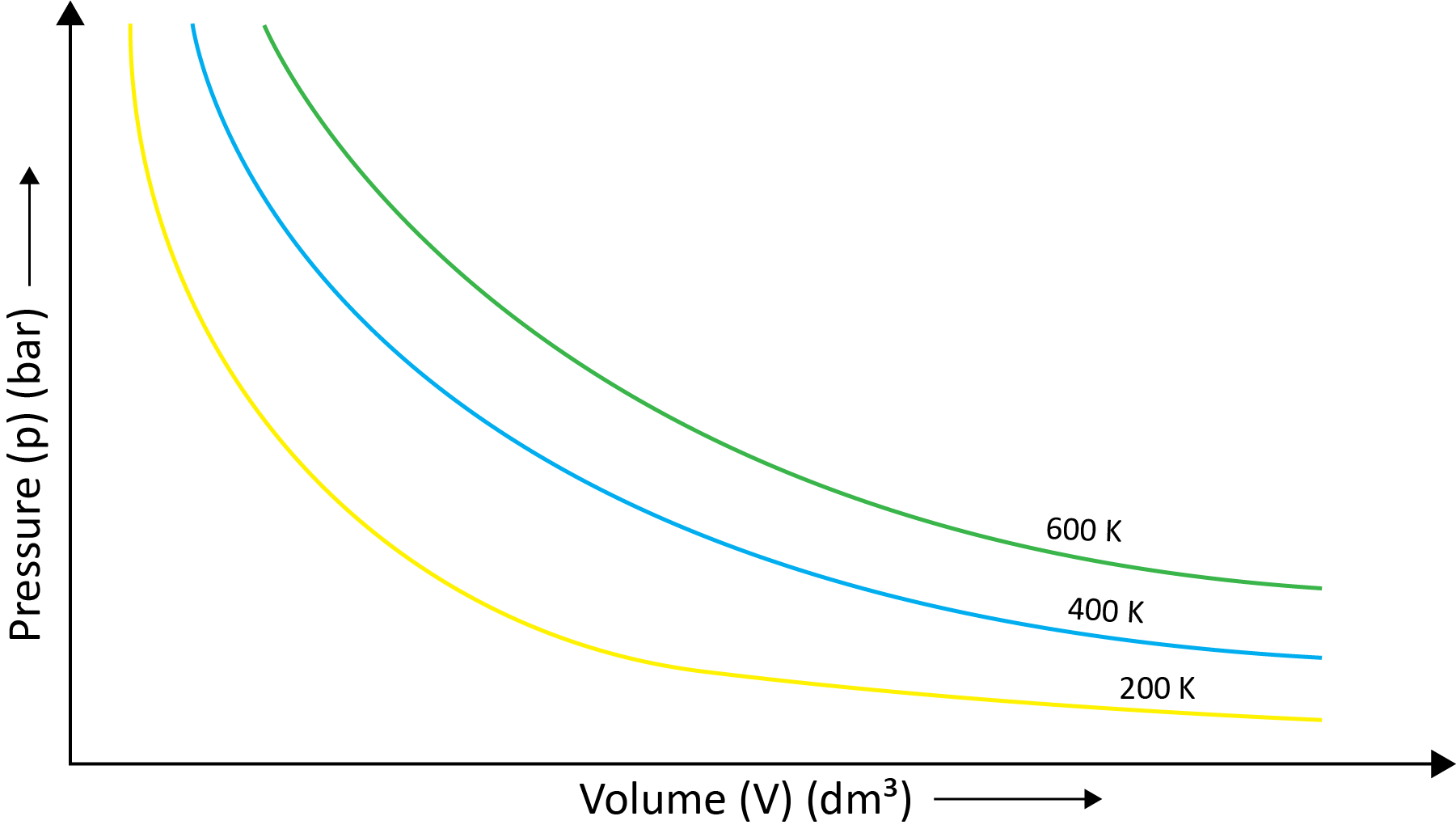
(b) Charles’ Law (Temperature - Volume Relationship)
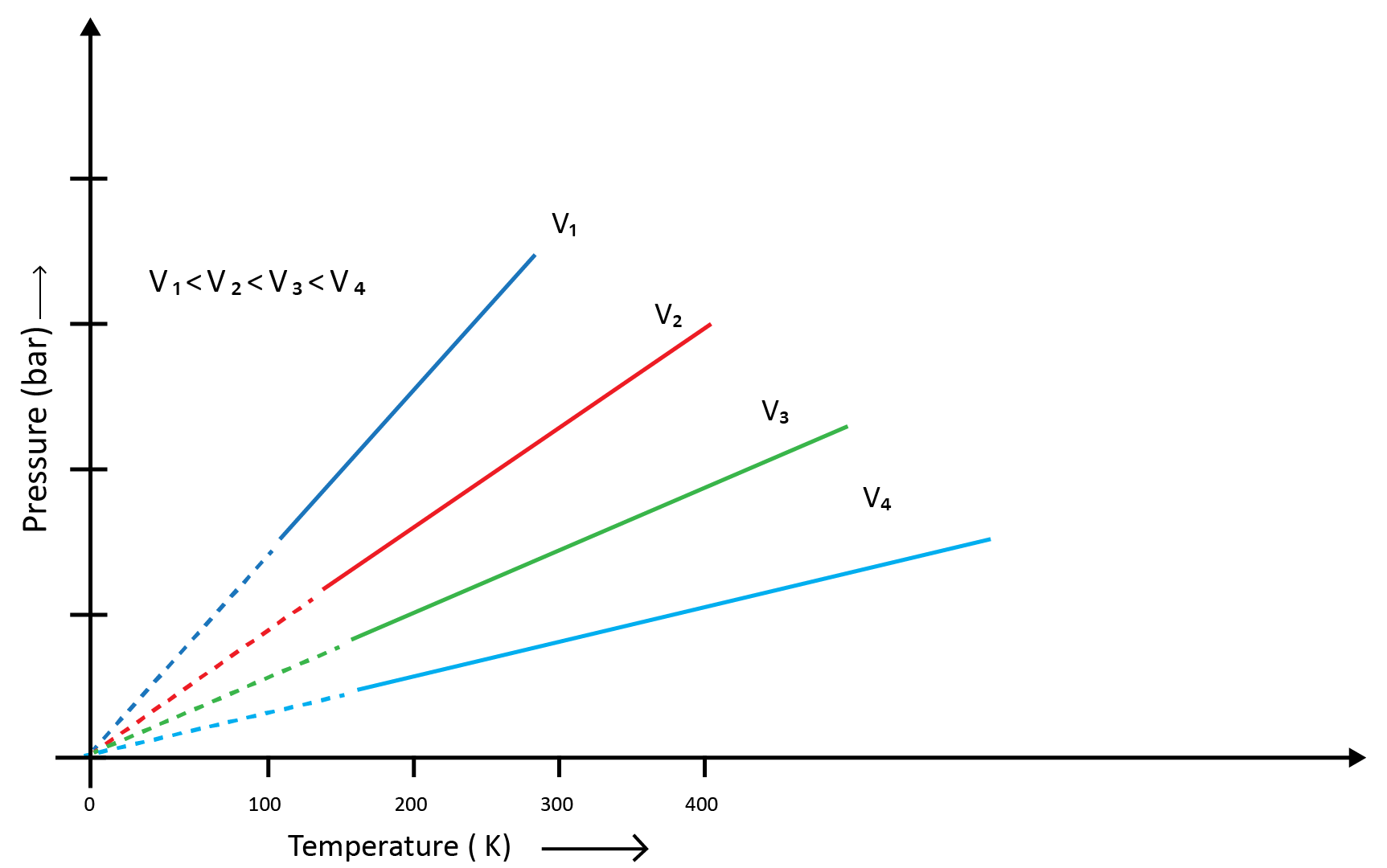
Charles and Gay Lussac individually conducted various gas experiments in order to advance hot air balloon technology. Their research revealed that the volume of a gas increases with increasing temperature and decreases with cooling for a fixed mass of a gas at constant pressure. The mathematical expression can be given as at the constant volume and pressure- V1/T1=V2/T2
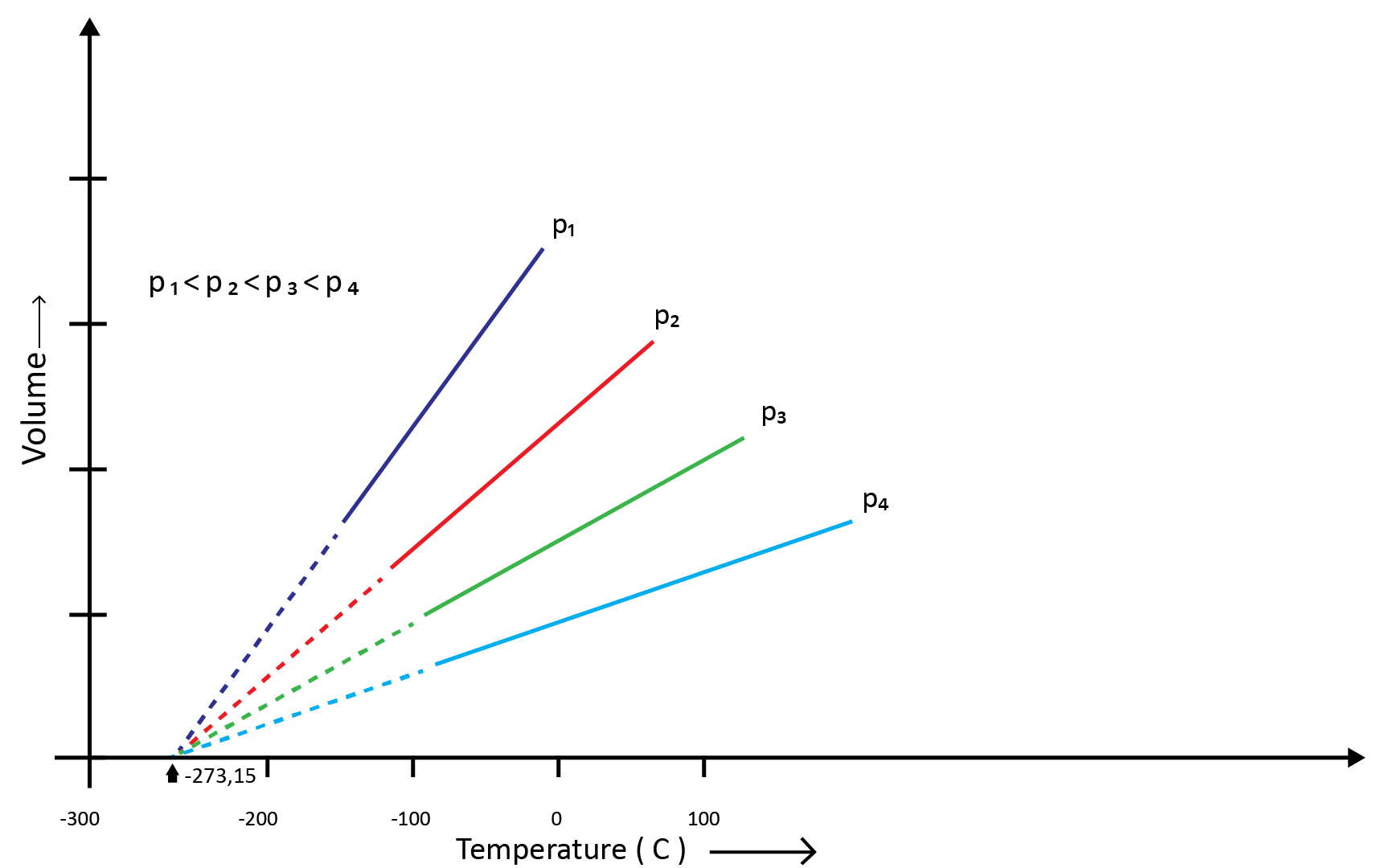
(c) Gay Lussac’s Law (Pressure-Temperature Relationship)
Joseph Gay Lussac established the mathematical link between pressure and temperature, which is known as Gay Lussac's law. It claims that the pressure of a fixed amount of gas varies directly with the temperature at constant volume. Mathematically,
P1/T1=P2/T2
Boyle's and Charles' laws can be used to draw this relationship. The above graph of pressure vs. temperature (Kelvin) at constant molar volume. Isochore refers to each line in the above graph.
(d) Avogadro Law (Volume - Amount Relationship)
It says that under the same temperature and pressure, identical volumes of all gases contain the same number of molecules. This means that the volume of a gas is determined by the number of molecules in the gas, or the amount of gas, as long as the temperature and pressure remain constant. Mathematically- V1/n1=V2/n2
6. Ideal Gas Equation
The three laws ( Boyle's law, Charles law, Avogadro's law) that we've learned so far can be merged into a single equation known as the ideal gas equation-pV=nRT
R is called gas constant. It is the same for all gases. Hence, it is also called Universal Gas Constant.
(a) Density and Molar Mass of a Gaseous Substance
The relation is written below-
d/M = P/RT
where d is the density.
(b) Dalton’s Law of Partial Pressures
John Dalton drafted the legislation in 1801. It asserts that the overall pressure exerted by a mixture of non-reactive gases is equal to the sum of individual gas partial pressure.
7. Kinetic Theory and Molecular Speeds
Gas molecules are constantly moving. They crash with each other and the container's walls as they move. As a result, their speed changes and energy is redistributed. As a result, the speed and energy of all the gas molecules at any given time are not the same.
The true distribution of molecular speeds in gas is dependent on temperature and molecule mass, as demonstrated by Maxwell and Boltzmann.
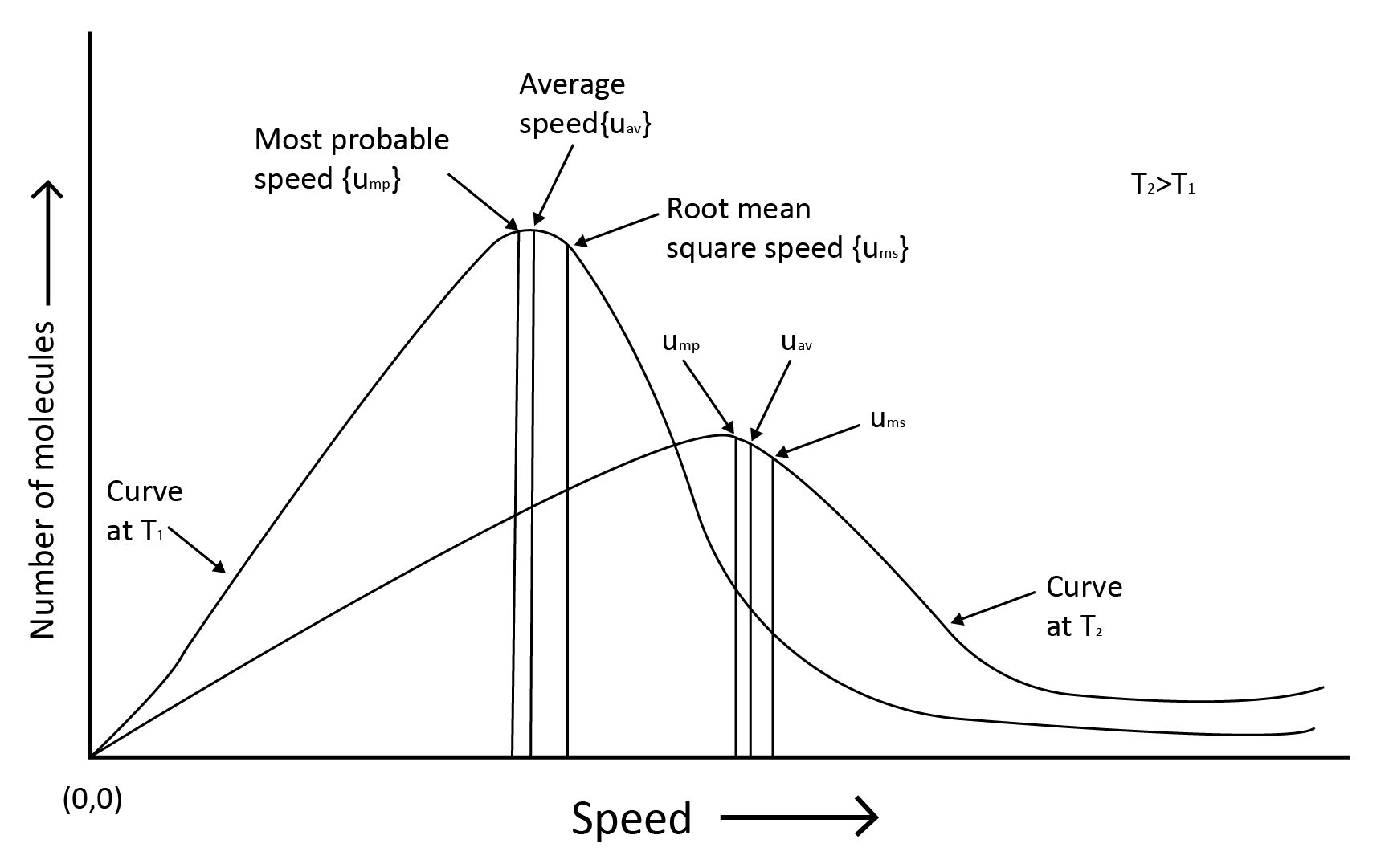
Root mean square speed, average speed and the most probable speed have the relationship: urms > uav > ump
8. Kinetic Theory of Gases
The postulates are given below-
1. Gases include a huge number of microscopic particles.
2. A gas exerts pressure on an object.
3. There is no kinetic energy loss.
4. Gas molecules are attracted to one another.
5. The molecule's kinetic energy is proportional to its absolute temperature.
6. The gaseous molecule's actual volume is relatively small.
7. Gaseous molecules are always moving.
8. Gravity has a greater impact on the movement of gaseous molecules.
9. Behaviour of Real Gases
When a pressure v/s volume plot is drawn, deviation from optimal behaviour is also visible. The experimental pressure vs volume plot (actual gas) and the theoretical pressure v/s volume plot (ideal gas) should be identical. This graph is shown below. The measured volume is clearly greater than the calculated amount at very high pressure.
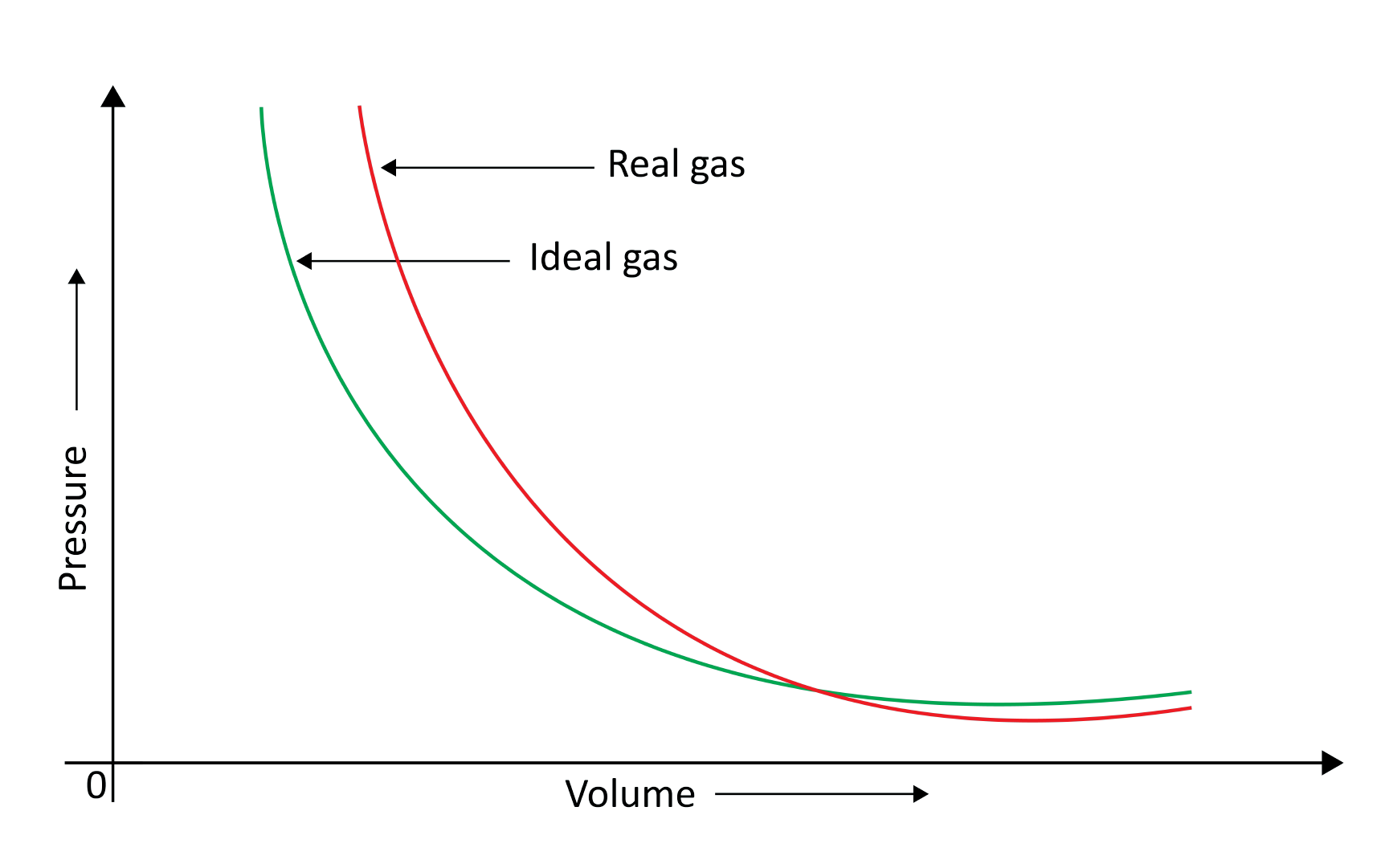
Because molecules interact with one another, real gases deviate from the ideal gas law.
At high pressures, gas molecules are extremely near to one another. Molecular interactions begin to take place. Molecules do not strike the container walls with full power at high pressure because they are pulled back by other molecules due to molecular attraction forces.
This has an impact on the pressure that molecules exert on the container's walls. As a result, the pressure exerted by the gas is lower than that of the ideal gas.
The compressibility factor Z, which is the ratio of product pV and nRT, can be used to calculate the departure from ideal behaviour.
Z =pV/nRT is a mathematical formula.
Because pV = nRT, Z = 1 for an ideal gas at all temperatures and pressures. The value of Z deviates from unity for gases that deviate from ideality. All of the gases presented have Z =1 and behave like ideal gases at very low pressures. At high pressure, all gases have a Z>1. Compressing these is more challenging. Most gases have Z<1 at moderate pressures.
10. Liquefaction of Gases
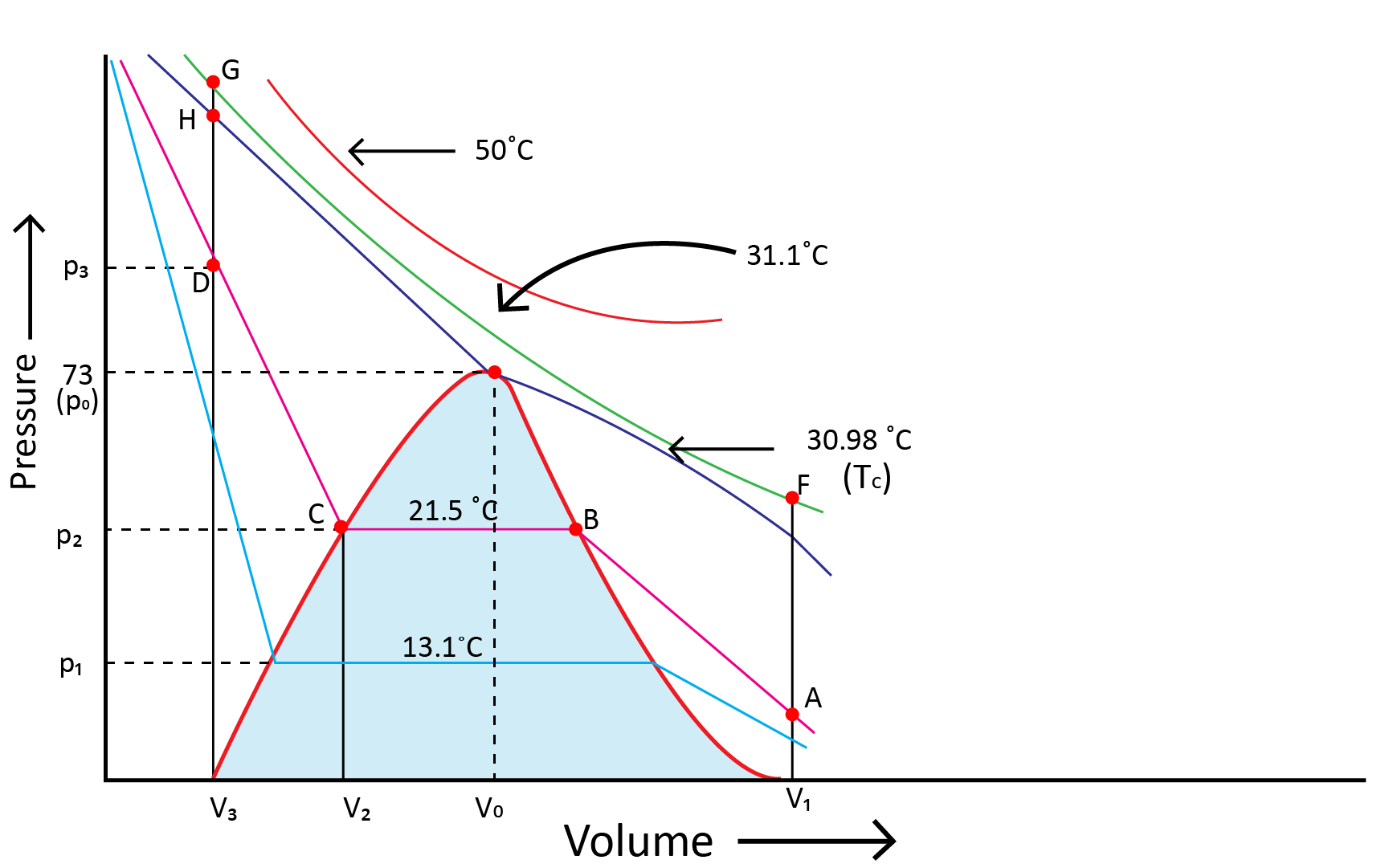
Andrews noted that isotherms at high temperatures resemble those of an ideal gas and that the gas cannot be liquefied even at very high pressure. The shape of the curve varies as the temperature drops, and the data shows a significant divergence from optimal behaviour. Carbon dioxide remains a gas up to 73-atmosphere pressure at 30.98°C (point E.) Liquid carbon dioxide occurs for the first time at 73 atmosphere pressure. The temperature of 30.98°C is known as the carbon dioxide critical temperature. This is the temperature at which liquid carbon dioxide can be found. It is gas above this temperature. Critical volume and critical pressure are terms used to describe the volume of one mole of a gas at a critical temperature.
11. Liquid State
In the liquid state, intermolecular forces are stronger than in the gaseous state. Liquid molecules are so close together that there is very little empty space between them, thus liquids are denser than gases under typical conditions. Liquid molecules are held together by intermolecular forces that are attractive. Because molecules do not split from one another, liquids have a defined volume.
(a) Vapour Pressure
If a partially filled evacuated container is a liquid in which a portion of the liquid evaporates in order to replenish the container's remaining volume with vapour. The liquid evaporates at first, and pressure is placed on the walls of a vessel by vapours The pressure inside the container (vapour pressure) rises. After that, It eventually settles into a steady state, and a state of equilibrium is established between the liquid and solid phases and phase of vaporisation At this point, the vapour pressure is high. Vapour pressure at this stage is called equilibrium vapour pressure or saturated vapour pressure.
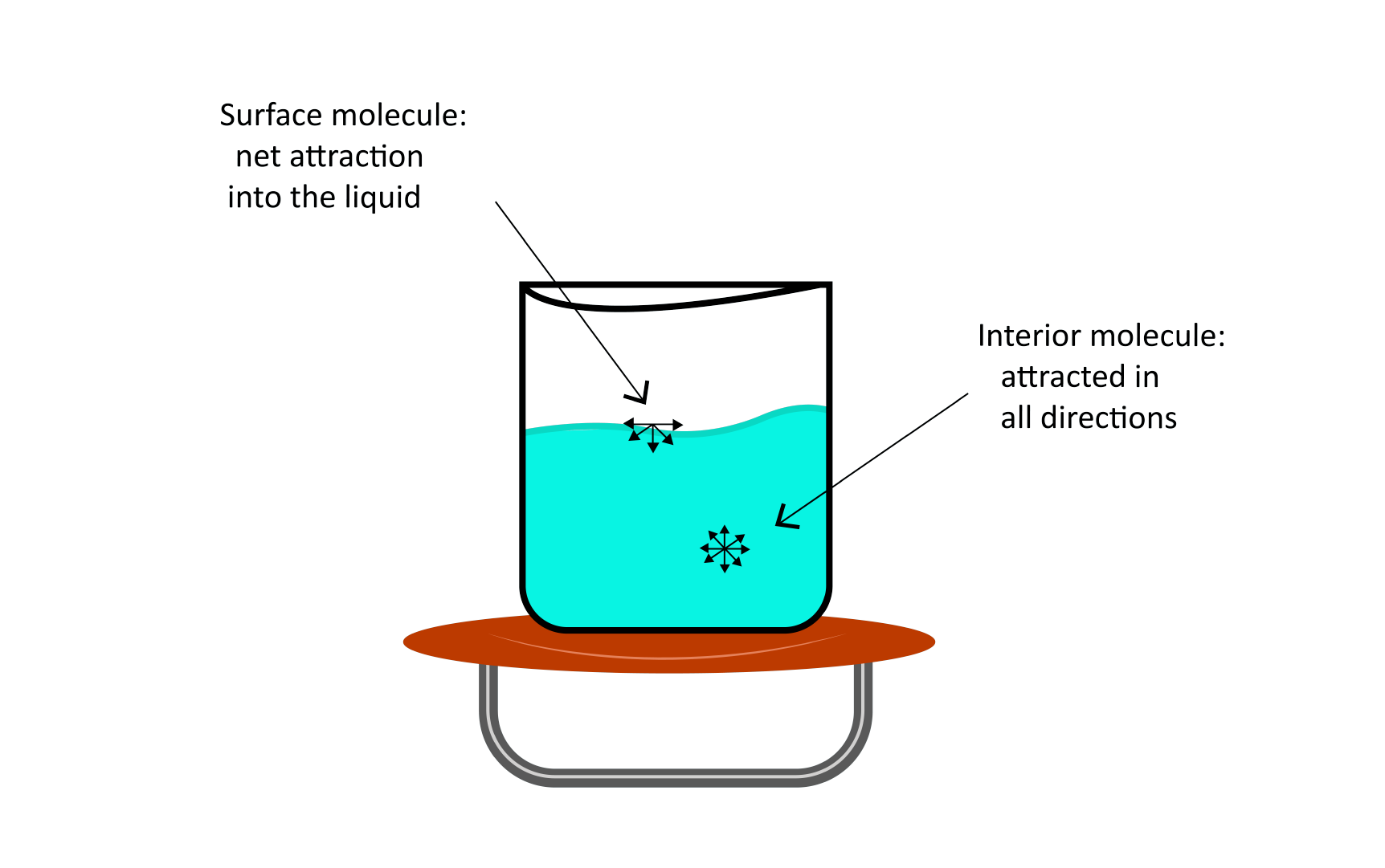
(b) Surface Tension
Surface tension is the tendency of liquid surfaces to shrink to the smallest possible surface area while they are at rest.
In the bulk of a liquid, a molecule is subjected to equal intermolecular forces from all sides. As a result, the molecule is not subjected to any net force. However, the net attractive force for a molecule on a liquid's surface is towards the liquid's interior.
(c) Viscosity
It is one of the distinguishing characteristics of liquids. Internal friction between layers of fluid as they slip past one another while liquid flows cause viscosity, which is a measure of flow resistance. Strong intermolecular interactions hold molecules together and prevent layers from moving past one another.
The viscosity of a liquid determines how slowly it moves. The van der Waals and hydrogen bonding forces are strong enough to generate significant viscosity. Glass is a viscous liquid with a high viscosity.
It has such a high viscosity that many of its properties are similar to those of solids.
Because at high temperatures, molecules have enormous kinetic energy and can overcome intermolecular interactions to glide past one another between layers, the viscosity of liquids lowers.
NEET Chemistry States of Matter Solved Examples
Example 1: Name the energy which arises due to the motion of atoms or molecules in a body. How is this energy affected when the temperature is increased?
Ans: The energy which arises due to the motion of atoms or molecules in a body is Thermal energy. It is a unit of measurement for the average kinetic energy of particles. It rises as the temperature rises.
The Key Point to Remember: Thermal energy is the energy that depends on the temperature.
Example 2: Name two intermolecular forces that exist between HF molecules in a liquid state.
Ans: The two intermolecular forces that exist between HF molecules in the liquid state are-
(i) Dipole-dipole interaction
(ii) Hydrogen – bonding
Key Point to Remember: When molecular interactions are weak, molecules do not stick together to form a liquid or solid until the temperature is lowered to minimise thermal energy. There are different types of forces between particles.
Previous Year Questions From NEET Papers
Question 1: At constant temperature the product of pressure and volume of a given amount of a gas is constant this is-
(a) Gay-Lussac law
(b) Charles’ law
(c) Boyle’s law
(d) None of these
Solution: According to Boyle’s law, the product of volume and pressure of a given mass of gas is constant at a constant temperature as PV= constant.
Hence, option (b) is the correct answer.
Trick: From all of the gas laws, Boyle's law is one of them in that temperature is constant as pressure is inversely proportional to volume.
Question 2: The kinetic theory of gases predicts that the total kinetic energy of gas depends on-
(a) Pressure of the gas
(b) Temperature of the gas
(c) Volume of the gas
(d) Pressure, temperature and volume of the gas
Solution: The kinetic theory of gases predicts that total kinetic energy of gas depends on the temperature of the gas. Therefore, option (b) is the correct answer.
Trick: The kinetic theory of the gases is only dependent on the temperature of the gas as it is one of the postulates of the kinetic theory of gases.
Question 3: Gases deviate from ideal behaviour because molecules-
(a) Are colourless
(b) Are spherical
(c) Attract each other
(d) Have high speeds
Solution: Gases deviate from ideal behaviour because molecules attract each other. Therefore, option (c) is the correct answer.
Trick: Real gases deviate from the ideal gas behaviour because in this there is a force of attraction between the particles.
Practice Questions
1. If the temperature is doubled, the average velocity of a gaseous molecule increases by-
(a) 4
(b) 1.4
(c) 2
(d) 2.8
Ans: (b)1.4
Question 2: Dominance of strong repulsive forces among the molecule of the gas:
(a) depends on Z and indicates that Z = 1
(b) depends on Z and indicates that Z > 1
(c) depends on Z and indicates that Z<1
(d) is independent of Z
Ans: (b)
Understanding the Importance of Learning the Chapter "States of Matter" for NEET:
The chapter "States of Matter" holds a pivotal role in the NEET (National Eligibility cum Entrance Test) syllabus and is of great significance in preparing aspiring medical students. Here are some key reasons why this chapter is essential for NEET:
Foundation for Thermodynamics: States of Matter introduces fundamental concepts such as the gas laws (Boyle's, Charles's, Avogadro's laws), the kinetic theory of gases, and thermodynamics. A strong grasp of these concepts is essential for the later chapters in thermodynamics and chemical equilibrium.
Application in Organic Chemistry: The knowledge of states of matter is crucial when it comes to understanding various physical properties and behaviors of gases, liquids, and solids. It aids in comprehending phase changes and intermolecular forces, which are significant in organic chemistry and biochemistry.
Biological Relevance: Aspiring medical professionals need to understand the behavior of gases and liquids in biological systems. This knowledge is vital for comprehending processes like respiration, digestion, and circulation, which are covered in biology and physiology.
Influence on Solubility: Understanding the states of matter is essential for comprehending solubility and colligative properties. This knowledge is valuable for the study of solutions, which has extensive applications in pharmacy and medicine.
Clinical and Pharmaceutical Applications: Medical professionals should understand the behavior of drugs, anesthetics, and other pharmaceuticals in different states of matter, especially when they enter the human body. This chapter provides the foundation for comprehending drug delivery systems.
Preparation for General Chemistry: States of Matter is a stepping stone for general chemistry and physical chemistry. A strong foundation in this chapter ensures a smoother transition into more advanced chemistry topics.
Scoring Potential: The NEET exam may contain questions directly or indirectly related to this chapter. A solid understanding of states of matter can help students score valuable marks in the examination.
Conclusion
Solid, liquid, or gaseous matter can exist in one of three states. Solid matter is made up of very closely packed particles. The particles in a solid do not have the freedom to move around. Liquid matter is made up of particles that are more loosely packed. It will take the shape of the container in which it is contained. Within a liquid, particles can move about, yet they are packed densely enough to preserve volume. Gaseous stuff is made up of particles that are packed so loosely that it has no defined shape or volume. Compressing a gas is possible.
Chemistry States of Matter Chapter - Chemistry NEET

 Share
ShareFAQs on Chemistry States of Matter Chapter - Chemistry NEET
1. What are the three states of matter that most people are familiar with?
The three states of matter have one thing in common: they are all made up of tiny, little particles. They have a defined mass and can occupy a certain amount of space. In each of these three states, there is a volume. The degree of attraction between atoms is strongest in these three states.
2. Is it possible to generate matter?
Furthermore, the first law of thermodynamics states that the total amount of energy in a closed system cannot be created or destroyed, but it can be changed from one form to another.
3. Why do distinct states of matter exist?
Intermolecular forces, the temperature of its surroundings and itself, and the density of the substance are all factors in the existence of distinct states of matter. The diagram below depicts how the shift between each state takes place (called Phase transitions).
4. Is states of matter important for NEET?
Yes, the states of matter is an important chapter for NEET. It is the foundation for understanding many other concepts in chemistry, such as thermodynamics, kinetics, and phase transitions. The chapter also covers important topics such as the ideal gas law, the kinetic molecular theory of gases, and the liquid state.
5. How is the change of state of matter used in everyday life?
The change of state of matter is used in many everyday applications. For example, cooking involves the change of state of water from liquid to gas and vice versa. Refrigeration and air conditioning work by using the latent heat of vaporization and condensation of water. The change of state of matter is also used in many industrial processes, such as distillation, crystallization, and chromatography.




















 Watch Video
Watch Video


