




Introduction to Thermodynamics
Thermodynamics is a branch of science that investigates heat, work, and temperature, as well as its connections to energy, radiation, and physical properties of the matter. The four laws of thermodynamics govern the behaviour of these values. It provides a quantitative description based on quantifiable macroscopic physical quantities, but statistical mechanics may be able to describe it in terms of microscopic elements.
Thermodynamics is the study of heat and energy with various physical transformations and chemical processes. It deals with the study of the energy transfer of a system with its surroundings. Energy in the form of heat can be absorbed or released by a reaction, and phase transitions like boiling and melting can do the same. The entropy of a reaction is used to assess whether it is favourable or unfavourable, spontaneous or nonspontaneous. It can also be used to calculate how much of each product and reactant is required in a complete reaction.
Thermodynamics is a crucial component of the JEE exam. The themes of an open system, closed system, isolated system, thermodynamic rules, thermodynamics formulae and so on are all important topics for the JEE exam. These topics are discussed in this article. This article is also used as thermodynamics chemistry notes for NEET aspirants. This article illustrates the types of questions that might be asked from this topic along with important thermodynamics notes.
Important Topics of Thermodynamics
Thermodynamic Processes.
First Law of Thermodynamics.
Second Law of Thermodynamics.
Third Law of Thermodynamics.
Enthalpy and Enthalpy Change.
Specific and Molar Heat Capacity.
Carnot Cycle.
Free Energy.
Exothermic Reaction.
Endothermic Reaction.
Heat of Reaction.
Important Definitions of Thermodynamics
State of a System and State Variables
Macroscopic qualities that specify the state of a system include state variables, state functions, and thermodynamic parameters.
The state qualities are only influenced by the system's starting and ending states.
It is unaffected by the method used to implement the change.
Thermodynamic Equilibrium
A system is said to have reached thermodynamic equilibrium when it displays no additional propensity to change its property over time.
The thermodynamic equilibrium criterion specifies that all the three equilibrium forms must occur simultaneously in a system.
Chemical Equilibrium: A system in which the system's composition is continuous and definite.
Mechanical Equilibrium: There is no chemical interaction between the system's components or between the system and its surroundings.
Maintain constant pressure in order to succeed.
Thermal Equilibrium: It is conceivable if the temperature remains constant, that is if there's no heat transfer between the system and the surroundings.
Thermodynamic Processes
The transition of a thermodynamic system from one state to another is referred to as a thermodynamic process. This process covers a number of processes.
Isothermal Process
In this procedure, the process is carried out at a constant temperature. E = 0 follows from dT = 0.
Adiabatic Process
In this method, there is no heat exchange between the system and its surroundings.
With dQ = 0 and insulated borders, the system is thermally isolated.
Isobaric Process
Throughout the shift in the process, the pressure remains constant, i.e., dP = 0.
For example, when water boils, it produces steam, and when it freezes, it produces ice.
Isochoric Process
Throughout the shift in the process, the volume remains constant, i.e., dV = 0.
For example, when a gasoline-air mixture is burned in a car engine, the temperature and pressure of the gas inside the engine rise. Meanwhile, the gas volume remains constant.
Cyclic Process
When a system goes through a succession of procedures before returning to its original state, it is called a cyclic process.
For a cyclic process, dE = 0 and dH = 0.
Reversible Process
Reversible process in thermodynamics is the process that is reversible in nature. But, while reversing the process there should not any changes made to the environment by system or its surroundings.
For example, springs that can be extended and get back to their original position. Electrolysis is the frictionless mobility of solids (with no resistance in the electrolyte).
Irreversible Process
When a process goes from its beginning condition to its final state in a single step in a fixed period of time and cannot be reversed, it is called an irreversible process.
The quantity of entropy grows in an irreversible process.
There are many irreversible processes in nature.
All the natural processes are irreversible in nature.
Examples of irreversible processes include the flow of electric current through a conductor with resistance.
Internal Energy
Every system has some amount of matter which possesses a definite amount of energy, hence the energy contained within the system is the energy contained within the system.
This energy is a state function that represents the sum of all energy forms (chemical, mechanical, electrical, etc.).
It is represented by the letter/symbol E.
First Law of Thermodynamics
It is also known as the law of energy conservation.
It states, “Energy can neither be created nor destroyed although it can be converted from one form into another.”
Change in internal energy = Heat added to the system + Work done on the system.
E2 - E1 = ΔE = q + wIt was conceived by Helmholtz and Robert Mayer.
The internal energy of a system is depleted when it does work (w) on the environment.
Change in internal energy = Heat added to the system – Work done by the system
ΔE = q + (- w) = q - w
Enthalpy and Enthalpy Change
The heat content of a system under constant pressure is called enthalpy.
The letter 'H' stands for Enthalpy.
From the first law of thermodynamics,
q = E + W ; q = E + PΔV
It is possible to provide a heat change at a constant pressure,
q = E + PΔV.
Heat may be replaced at enthalpy at constant pressure.
ΔH = E + PΔV.
The heat of reaction (in a chemical process) or the heat change under constant pressure is denoted by the letter H.
Specific Heat Capacity and Molar Heat Capacity
The amount of heat required to raise the temperature of one gram of a substance by one degree celsius is known as its specific heat capacity.
q = mcΔT; (cp = constant pressure/cv = constant volume)
The amount of heat required to raise the temperature of one mole of a substance by one degree celsius is known as its molar heat capacity.
Gases have a tendency to expand when heated under constant pressure, necessitating an additional energy source to raise their temperature by 1°C when necessary under constant volume conditions.
Therefore, Cp > Cv.
Cp = Cv + Work done in expansion; Work done in expansion is PΔV(=R); Cv = molar heat capacities at constant volume, Cp = molar heat capacities at constant pressure.
Relations between Cp and Cv.
Second Law of Thermodynamics
All of the limits imposed by the first law are removed by the second law of thermodynamics.
This law shows that spontaneous processes have a positive overall energy change.
The Carnot Cycle
Carnot cycle is a type of cyclic process in which the net work done equals the heat absorbed.
Thus, the bigger the temperature differential between the high and low temperature reservoirs, the more heat is generated, which is converted into work by the heat engine.
Thermodynamic efficiency = w/q2=(T2-T1)/T1.
Entropy
Entropy is a metric that measures the unpredictability or disorder of a system's molecules.
It's a metric for assessing the state of the thermodynamic system.
Sfinal - Sinitial = ΔS = qrev/T.
If heat is absorbed, then S = -ve and if heat is evolved, then, ΔS = -ve.
Free Energy
Gibbs free energy (G) is a unit of measurement for the maximum work done by a reversible process under constant pressure and temperature, also known as useful work done.
By subtracting the system's enthalpy from the product of absolute temperature and entropy, we can find Gibbs free energy, i.e., ΔG = ΔH - TΔS.
Third Law of Thermodynamics
This law states, “The entropy of all perfectly crystalline solids approaches zero at the temperature approaches absolute zero.”
Because entropy is a measure of disorder, a perfectly crystalline material has a perfect order of its constituent particles at absolute zero.
Exothermic and Endothermic Reactions
Exothermic reactions are those that occur as a result of heat energy evolution.
ΔH = Hp - Hr; Hp < Hr; ΔH = -ve.
Examples of Exothermic Reactions
Endothermic reactions are those that take place when heat energy is absorbed.
ΔH = Hp - Hr; Hp < Hr; ΔH = +ve.Examples of Endothermic Reactions
Heat of a Reaction or Enthalpy of a Reaction
The heat of a reaction, also known as reaction enthalpy, is the difference between the enthalpies of the products and reactants acquired after the reactants reacted entirely in a chemical process.
Enthalpy of reaction (heat of reaction) = ΔH = ΣHp - ΣHr.
Solved Examples from the Chapter
Example 1: The entropy of a system approaches a constant value as the temperature ______.
Increases
Decreases
Approaches absolute zero
None of the options
Solution:
When the temperature approaches absolute zero, the system approaches the constant value, according to the third rule of thermodynamics.
Hence, the correct answer is option (c) Approaches absolute zero.
Key point to remember: Third Law of Thermodynamics: The entropy of all perfectly crystalline solids approaches zero when the temperature approaches absolute zero
Example 2: Which of the following statements is correct?
An open system is one in which reacting species are present in a covered beaker.
In a closed system, there is an exchange of energy and matter between the system and its surroundings.
The presence of reactants in a closed vessel made up of copper is an example of a closed system.
A closed system is defined as the presence of reactants in a thermos flask or any other closed insulated vessel.
Solution:
In a closed system, there is no interchange of substances (for example, the existence of reactants in a closed vessel made of a conducting material like copper).
But there is an exchange of energy between the system and its surroundings.
Hence, the correct answer is (c). The presence of reactants in a closed vessel made up of copper is an example of a closed system.
Key point to remember: Definition of a closed system. In a closed type of system, there can be an interchange of energy with its surroundings in the form of heat, work, or radiation, but not matter.
Solved Examples from Previous Year Questions
Question 1: The internal energy change in a system that has absorbed 2 kcals of heat and done 500 J of work is:
6400 J
5400 J
7900 J
8900 J
Solution:
According to the first law of thermodynamics,
Q = ΔU + W,
∴ ΔU = Q – W
= 2 × 4.2 × 1000 – 500
= 8400 –500
= 7900 J
Hence, the correct answer is option (c) 7900 J
Trick: First Law of thermodynamics: Change in internal energy = Heat added to the system +Work done on the system.
Question 2: A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then :
Compressing the gas isothermally will require more work to be done.
Compressing the gas through an adiabatic process will require more work to be done.
Compressing the gas isothermally or adiabatically will require the same amount of work.
Which of the case (whether compression through isothermal or through adiabatic process) requires more work will depend upon the atomicity of the gas
Solution:
The external work done is the negative of the area with the volume axis from the figure given below:
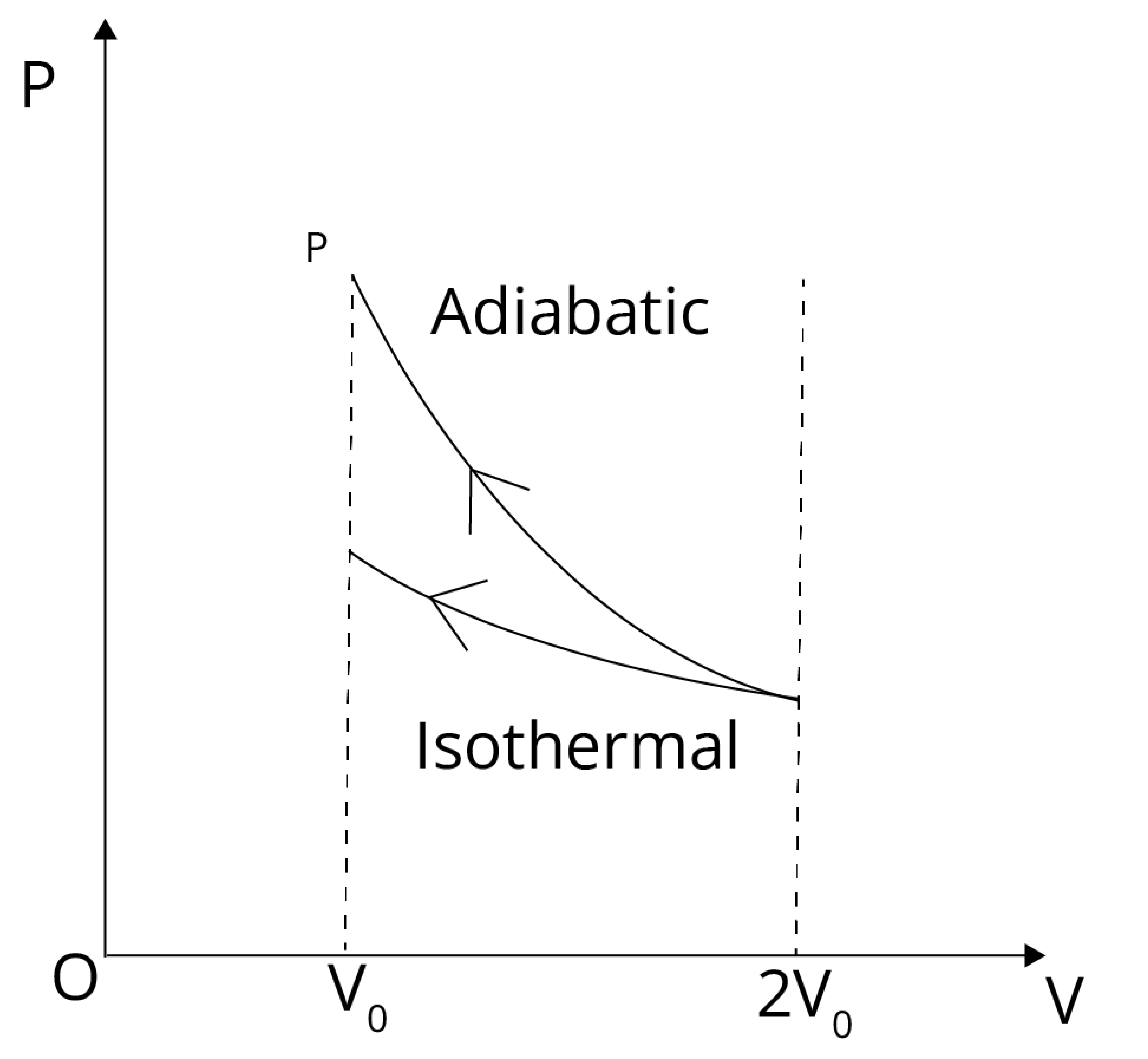
Also, the adiabatic work done is greater than the isothermal work done.
Work Done (adiabatic) > Work Done (isothermal).Hence, the correct answer is (b). Compressing the gas through an adiabatic process will require more work to be done.
Trick: The isothermal process is carried out at a steady temperature in this procedure. E = 0 is the result of dT = 0. There is no heat exchange between the system and its surroundings in an adiabatic process. The adiabatic system is thermally isolated with dQ = 0 and insulated borders.
Question 3: An ideal gas is compressed to half its initial volume by means of several processes. Which of the processes results in the maximum work done on the gas?
Isobaric
Isochoric
Isothermal
Adiabatic
Solution:
Because the area under the curve is the greatest for an adiabatic process, the work done on the gas (W = PdV) is greatest for an adiabatic process.
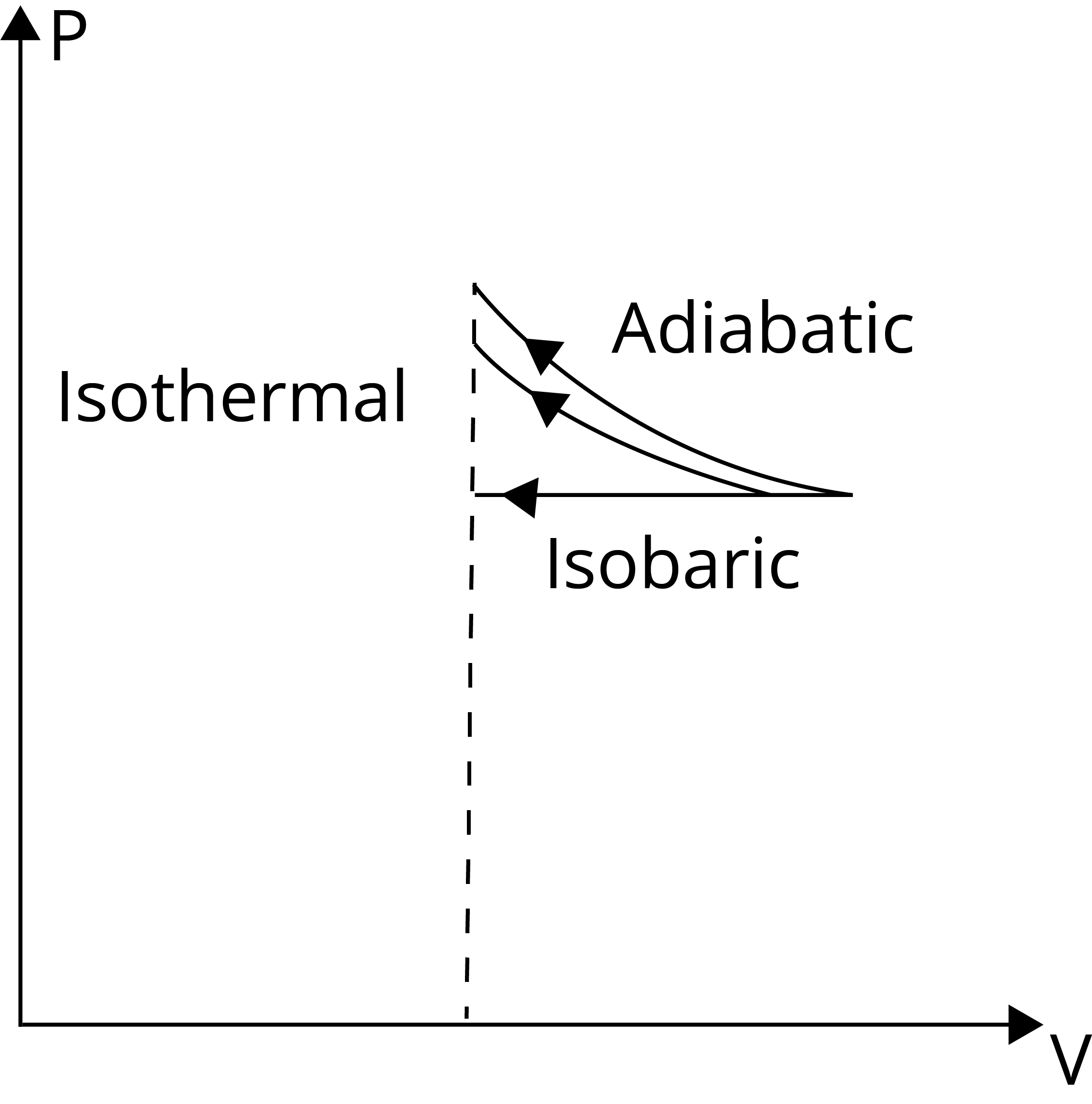
Hence, the correct answer is (d) Adiabatic.
Trick: There is no heat exchange between the system and its surroundings in the adiabatic process. The system is thermally isolated with dQ = 0 and insulated borders.
Practice Questions
Question 1: The entropy of a flawless crystal is 0 at absolute zero. In thermodynamics it is referred to as:
The First Law
The Second Law
The Third Law
None of the Above.
Answer: (c) The Third Law.
Question 2: The direction of any chemical reaction at constant pressure and temperature is one in which the ______ decreases.
Entropy
Gibbs free energy
Enthalpy
None of the above
Answer: (b) Gibbs free energy.
Conclusion
Thermodynamics is the study of how one body interacts with another in terms of heat and work. In this context, a system is defined as a quantity of substance or a location in space under investigation. Anything that exists outside of the system is referred to as the environment.
NEET Chapter Page - Thermodynamics

 Share
ShareFAQs on NEET Chapter Page - Thermodynamics
1. How many questions are asked from Thermodynamics in NEET?
About 1-2 questions from this chapter are asked for NEET. It corresponds to around 4-8 marks in the NEET exam. Generally, questions from this chapter are of moderate difficulty.
2. What are the important topics that must not be missed from Thermodynamics for the NEET exam?
Compared to other chapters, questions asked from this chapter thermodynamics for NEET exam are not too difficult. Frequent questions are asked related to efficiency of heat engines and laws of thermodynamics. Students must go through thermodynamics notes before starting solving numerical problems.
3. How to get a good score in the NEET exam to get into the best medical colleges?
NEET exam is not a very tough exam to crack. Getting around 600 marks can get you a seat in one of the best medical colleges. All you have to do is learn the concepts and solve all the questions in NCERT CBSE class 11 and class 12 along with solving last 20 years previous year NEET question papers.




















 Watch Video
Watch Video


