
1-Methylcyclohexane is allowed to react with ${{B}_{2}}{{H}_{6}}$. The product is then treated with ${{H}_{2}}{{O}_{2}}$ and NaOH. The product formed is:
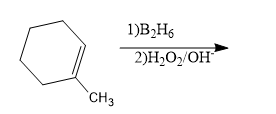
A) 1-methyl cyclohexanol
B) 2-methyl cyclohexanol ${{B}_{2}}{{H}_{6}}$
C) Methyl cyclohexane
D) Cyclohexanol

Answer
565.5k+ views
Hint:. The reaction involved is the hydroboration – oxidation reaction.
- The nucleophile added in this reaction is $O{{H}^{-}}$.
Complete step by step answer:
Here 1-methylcyclohexane reacts with ${{B}_{2}}{{H}_{6}}$ and the obtained product is treated with ${{H}_{2}}{{O}_{2}}$ and NaOH, we have to predict the product obtained.
- Here, we know that while the reactant, 1-methyl cyclohexane when reacts with diborane, hydroboration reaction takes place and the obtained product undergoes oxidation due to the reaction with ${{H}_{2}}{{O}_{2}}$ and NaOH.
- Hydroboration- oxidation reaction is the reaction in which an alkene undergoes a reaction with diborane and which is oxidized to give alcohol.
So simply we can say that alkene will be converted to alcohol during hydroboration –oxidation.
- In hydroboration –oxidation reaction product will be formed according to the anti-Markovnikov's rule i.e. the nucleophile will attack the reactant in the position of carbon having higher number of hydrogen or can be said as the nucleophile attack the least substituted carbon.
Now let’s see this reaction.
- First we should know the nucleophile and electrophile attacking. By the process of hydrolysis $O{{H}^{-}}$ and ${{H}^{+}}$ ions are formed. So${{H}^{+}}$ is the electrophile and $O{{H}^{-}}$ is the nucleophile formed.
Now where do the nucleophile attacks?
- We know that the nucleophile will attack the least substituted C or C with more hydrogen. Let’s check the number of H across the carbons with double bonds.
- The C attached to the methyl group ($-C{{H}_{3}}$) is having no H, since the valency of C is 4 and the other C is having one hydrogen. So the nucleophile will attack that carbon.
- And the electrophile i.e. ${{H}^{+}}$ will attach with the methyl group substituted C.
Hence the product will be:
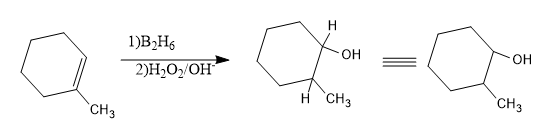
Now let’s write the name of the product obtained.
- In the obtained cyclic structure, numbering should start from the C attached to –OH group and a methyl group is attached to the second carbon, hence the name will be 2-methyl cyclohexanol.
So, the correct answer is “Option B”.
Note: We should know whether the product is formed through Markonikov’s rule or through Anti-Markovnikov's rule.
- In the naming of the product cyclohexanol is given instead of hexanol, since it is a cyclic structure, so a prefix –cyclo should be given while naming. And the –ol comes as the suffix, since the functional group is alcohol.
- The nucleophile added in this reaction is $O{{H}^{-}}$.
Complete step by step answer:
Here 1-methylcyclohexane reacts with ${{B}_{2}}{{H}_{6}}$ and the obtained product is treated with ${{H}_{2}}{{O}_{2}}$ and NaOH, we have to predict the product obtained.
- Here, we know that while the reactant, 1-methyl cyclohexane when reacts with diborane, hydroboration reaction takes place and the obtained product undergoes oxidation due to the reaction with ${{H}_{2}}{{O}_{2}}$ and NaOH.
- Hydroboration- oxidation reaction is the reaction in which an alkene undergoes a reaction with diborane and which is oxidized to give alcohol.
So simply we can say that alkene will be converted to alcohol during hydroboration –oxidation.
- In hydroboration –oxidation reaction product will be formed according to the anti-Markovnikov's rule i.e. the nucleophile will attack the reactant in the position of carbon having higher number of hydrogen or can be said as the nucleophile attack the least substituted carbon.
Now let’s see this reaction.
- First we should know the nucleophile and electrophile attacking. By the process of hydrolysis $O{{H}^{-}}$ and ${{H}^{+}}$ ions are formed. So${{H}^{+}}$ is the electrophile and $O{{H}^{-}}$ is the nucleophile formed.
Now where do the nucleophile attacks?
- We know that the nucleophile will attack the least substituted C or C with more hydrogen. Let’s check the number of H across the carbons with double bonds.
- The C attached to the methyl group ($-C{{H}_{3}}$) is having no H, since the valency of C is 4 and the other C is having one hydrogen. So the nucleophile will attack that carbon.
- And the electrophile i.e. ${{H}^{+}}$ will attach with the methyl group substituted C.
Hence the product will be:

Now let’s write the name of the product obtained.
- In the obtained cyclic structure, numbering should start from the C attached to –OH group and a methyl group is attached to the second carbon, hence the name will be 2-methyl cyclohexanol.
So, the correct answer is “Option B”.
Note: We should know whether the product is formed through Markonikov’s rule or through Anti-Markovnikov's rule.
- In the naming of the product cyclohexanol is given instead of hexanol, since it is a cyclic structure, so a prefix –cyclo should be given while naming. And the –ol comes as the suffix, since the functional group is alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




