
2-chloro-2-methylpentane on reaction with sodium methoxide in methanol yields:
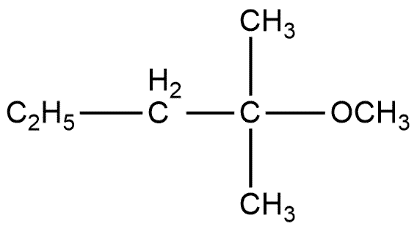
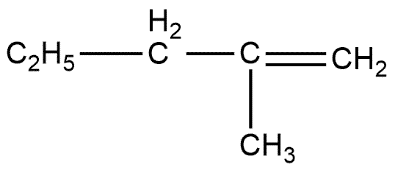
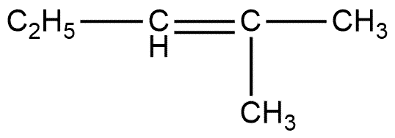
(a) $ \qquad $ (b) $ \qquad $ (c)
A. a and c
B. c only
C. a and b
D. All of these



Answer
577.8k+ views
Hint: 2-chloro-2-methylpentane is five membered long chain of carbon atoms here penta means 5 atoms of carbon and on 2 position i.e. on 2nd carbon atom methyl and chloro group is present due to which it named as 2-chloro-2-methylpentane.
Complete answer:
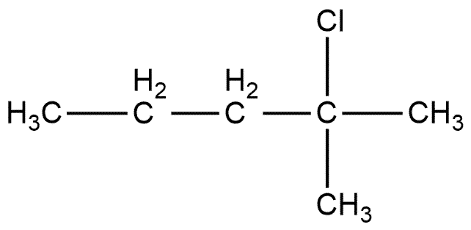
2-chloro-2-methylpentane is shown as:
Sodium methoxide is a chemical compound shown by the formula\[C{{H}_{3}}ONa\]. It is a white solid substance which is formed by the deprotonation of methanol. It is used as a reagent in many industries. It is also a dangerously caustic base. It is generally prepared by treating methanol i.e. \[C{{H}_{3}}OH\] with Na and the process is highly exothermic in nature and the solution prepared is colorless. The structure and the basicity of sodium methoxide in solution generally depends on the solvent. It is a significantly stronger base in DMSO as in this it is more fully ionized and free from hydrogen bonding.
2-chloro-2-methylpentane on reaction with sodium methoxide in methanol yields multiple products which can be shown as:
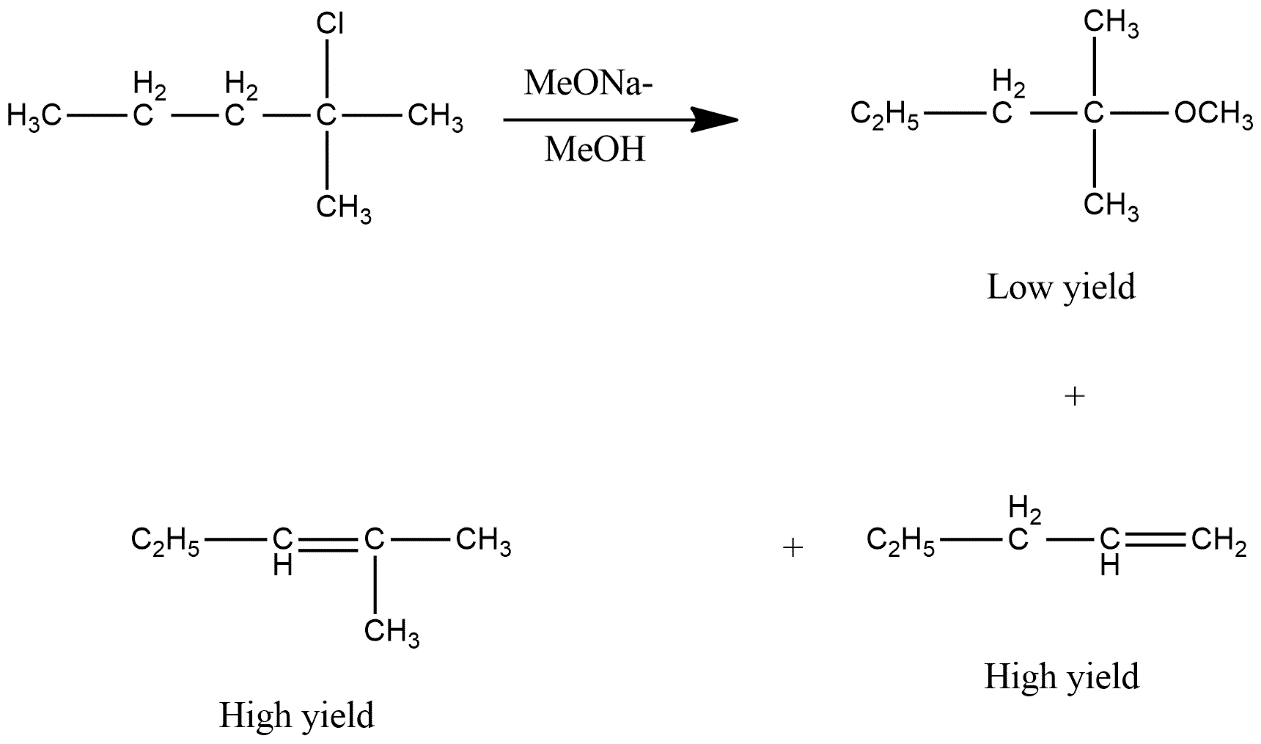
Above reaction shows that all options are correct. The only difference is that option A product is in low amount i.e. gives low yield product while other two are present in more amount. Thus we can say that option D is the correct answer.
Note:
Solid sodium methoxide is not much stable in air and easily degrades into a number of other sodium salts when exposed to air. This instability can be prevented by storing sodium methoxide under an inert atmosphere. Sodium methoxide is highly caustic and reacts with water to give methanol which is toxic and volatile in nature.
Complete answer:
2-chloro-2-methylpentane is shown as:

Sodium methoxide is a chemical compound shown by the formula\[C{{H}_{3}}ONa\]. It is a white solid substance which is formed by the deprotonation of methanol. It is used as a reagent in many industries. It is also a dangerously caustic base. It is generally prepared by treating methanol i.e. \[C{{H}_{3}}OH\] with Na and the process is highly exothermic in nature and the solution prepared is colorless. The structure and the basicity of sodium methoxide in solution generally depends on the solvent. It is a significantly stronger base in DMSO as in this it is more fully ionized and free from hydrogen bonding.
2-chloro-2-methylpentane on reaction with sodium methoxide in methanol yields multiple products which can be shown as:

Above reaction shows that all options are correct. The only difference is that option A product is in low amount i.e. gives low yield product while other two are present in more amount. Thus we can say that option D is the correct answer.
Note:
Solid sodium methoxide is not much stable in air and easily degrades into a number of other sodium salts when exposed to air. This instability can be prevented by storing sodium methoxide under an inert atmosphere. Sodium methoxide is highly caustic and reacts with water to give methanol which is toxic and volatile in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




