
2-hexyne gives trans-2-hexene on treatment with
(A)$Pt/H_2$
(B)Li/${\rm{N}}{{\rm{H}}_{\rm{3}}}$
(C)${\rm{Pd}}/{\rm{BaS}}{{\rm{O}}_{\rm{4}}}$
(D)${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$
Answer
558.6k+ views
Hint:We know that a trans isomer is a isomer in which functional groups are present at the opposite side of the carbon chain and a cis isomer is a isomer in which functional groups are present at the same direction of carbon chain.
Complete step by step answer:
Here, 2-hexyne undergoes reduction to form trans-2-hexene.
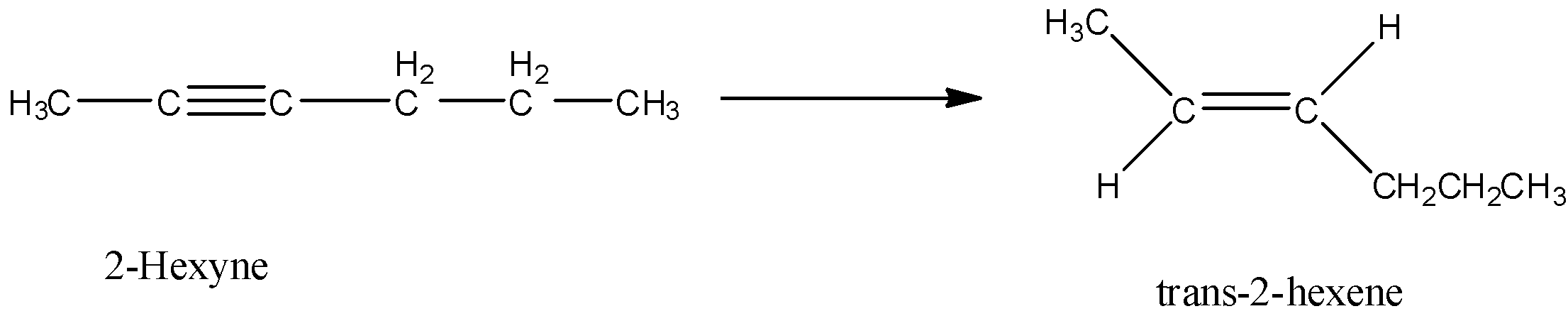
Let’s discuss the given options one by one.
Option A is $Pt/H_2$. Hydrogenation in presence of platinum reduces an alkyne to alkene. In this reduction reaction, hydrogen atoms are added by the trans addition, that means, on the opposite side of the double bond. So, when 2-hexyne undergoes reaction with $Pt/H_2$, trans-2-hexene is formed.
Option B is Li/${\rm{N}}{{\rm{H}}_{\rm{3}}}$. Li/${\rm{N}}{{\rm{H}}_{\rm{3}}}$ is also called a Lindlar catalyst. This catalyst is used to convert alkyne to alkene. But the hydrogen added is in cis manner. So, a cis-isomer obtained in the reaction. So, the formation of trans-2-hexene is not possible by the Lindlar catalyst.
Option C is ${\rm{Pd}}/{\rm{BaS}}{{\rm{O}}_{\rm{4}}}$. ${\rm{Pd}}/{\rm{BaS}}{{\rm{O}}_{\rm{4}}}$ is also a reducing agent. It reduces an alkyne to an alkene. The alkene formed is cis-isomer. So, the formation of trans-2-hexene is not possible by ${\rm{Pd}}/{\rm{BaS}}{{\rm{O}}_{\rm{4}}}$.
Option D is ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$(lithium aluminium hydride). Lithium aluminium hydride is a strong reducing agent. It can reduce polar multiple bonds such as C=O. It reduces aldehydes to primary alcohols, carboxylic acids and esters to primary alcohols. The reduction of isolated non-polar multiple bonds cannot be possible by ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$. The reduction of alkynes by ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$ is possible if an alcohol group is present nearby. So, the formation of trans-2-hexene is not possible by ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$.
Hence, the correct answer is option A.
Note:
It is to be noted that oxidizing agents are those chemical species that cause oxidation such as oxidation of alcohol to aldehyde, oxidation of aldehyde to carboxylic acid. Some common oxidizing agents are hydrogen peroxide, oxygen, chlorine etc.
Complete step by step answer:
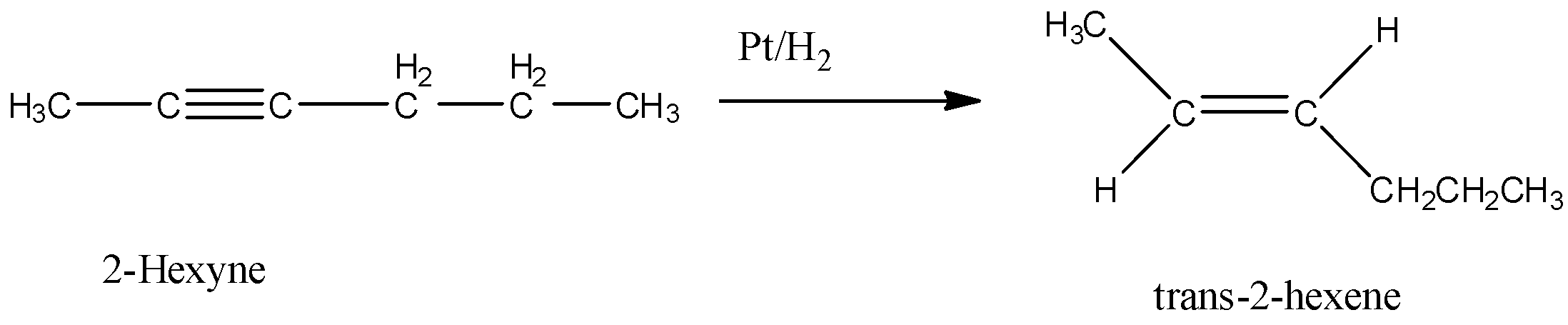
Here, 2-hexyne undergoes reduction to form trans-2-hexene.

Let’s discuss the given options one by one.
Option A is $Pt/H_2$. Hydrogenation in presence of platinum reduces an alkyne to alkene. In this reduction reaction, hydrogen atoms are added by the trans addition, that means, on the opposite side of the double bond. So, when 2-hexyne undergoes reaction with $Pt/H_2$, trans-2-hexene is formed.

Option B is Li/${\rm{N}}{{\rm{H}}_{\rm{3}}}$. Li/${\rm{N}}{{\rm{H}}_{\rm{3}}}$ is also called a Lindlar catalyst. This catalyst is used to convert alkyne to alkene. But the hydrogen added is in cis manner. So, a cis-isomer obtained in the reaction. So, the formation of trans-2-hexene is not possible by the Lindlar catalyst.
Option C is ${\rm{Pd}}/{\rm{BaS}}{{\rm{O}}_{\rm{4}}}$. ${\rm{Pd}}/{\rm{BaS}}{{\rm{O}}_{\rm{4}}}$ is also a reducing agent. It reduces an alkyne to an alkene. The alkene formed is cis-isomer. So, the formation of trans-2-hexene is not possible by ${\rm{Pd}}/{\rm{BaS}}{{\rm{O}}_{\rm{4}}}$.
Option D is ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$(lithium aluminium hydride). Lithium aluminium hydride is a strong reducing agent. It can reduce polar multiple bonds such as C=O. It reduces aldehydes to primary alcohols, carboxylic acids and esters to primary alcohols. The reduction of isolated non-polar multiple bonds cannot be possible by ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$. The reduction of alkynes by ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$ is possible if an alcohol group is present nearby. So, the formation of trans-2-hexene is not possible by ${\rm{LiAl}}{{\rm{H}}_{\rm{4}}}$.
Hence, the correct answer is option A.
Note:
It is to be noted that oxidizing agents are those chemical species that cause oxidation such as oxidation of alcohol to aldehyde, oxidation of aldehyde to carboxylic acid. Some common oxidizing agents are hydrogen peroxide, oxygen, chlorine etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




