
2-iodo cyclohexyl acrylate undergoes acetolysis. Which of the following statements is/are true.
A. Trans-isomer of the substrate undergoes acetolysis 670 times faster than that of its cis-isomer.
B. Cis-isomer of the substance undergoes cytolysis 670 times faster than that of its trans-isomer.
C. Both isomers undergo cytolysis with the same rate.
D. The enhanced rate of this nucleophilic substitution is due to neighbouring group precipitation.
Answer
573.9k+ views
Hint: Isomers are those compounds which have the same molecular formula or we can say that which have the same number of atoms but have different arrangement of atoms in space. They may or may not have similar physical and chemical properties.
Complete answer:
Acetolysis is the process of decomposition of an organic compound with the addition of elements of acetic acid at the point of decomposition This process is similar to hydrolysis and phosphorolysis. The cytolysis process is also known as osmotic lysis which occurs when a cell bursts due to an osmotic imbalance that has caused excess water to diffuse into the cell. It occurs in a hypotonic environment where water moves into the cell by osmosis and causes its volume to increase to the point where the volume exceeds the membrane's capacity and the cell bursts.
2-iodocyclohexyl acrylate undergoes acetolysis in which trans-2-iodo cyclohexyl acrylate undergoes acetolysis 670 times faster than the cis-isomer. The reaction of the trans-isomer proceeds through participation of neighbouring group i.e. iodo group while such neighbouring group participation by iodo group is not possible in the cis-isomer. Hence, cis-isomer undergoes the normal $S{{N}^{2}}$ mechanism. Mechanism can be shown as follows:
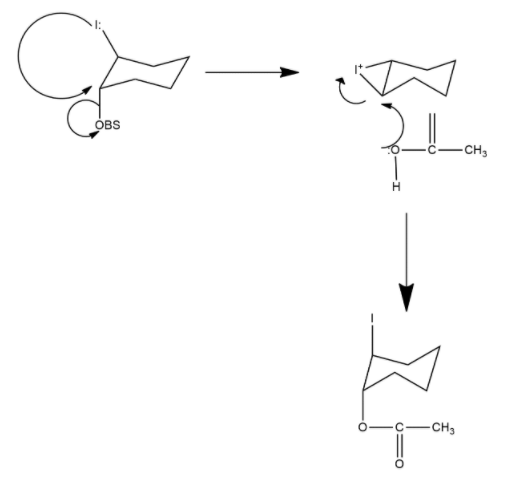
Hence we can say that option A is the correct answer.
Note:
$S{{N}^{2}}$ is generally a type of nucleophilic substitution reaction. It is one step reaction in which one bond is broken and one bond is formed synchronously. If the substrate under nucleophilic attack is chiral in nature then this often leads to inversion of configuration which is called as a Walden inversion.
Complete answer:
Acetolysis is the process of decomposition of an organic compound with the addition of elements of acetic acid at the point of decomposition This process is similar to hydrolysis and phosphorolysis. The cytolysis process is also known as osmotic lysis which occurs when a cell bursts due to an osmotic imbalance that has caused excess water to diffuse into the cell. It occurs in a hypotonic environment where water moves into the cell by osmosis and causes its volume to increase to the point where the volume exceeds the membrane's capacity and the cell bursts.
2-iodocyclohexyl acrylate undergoes acetolysis in which trans-2-iodo cyclohexyl acrylate undergoes acetolysis 670 times faster than the cis-isomer. The reaction of the trans-isomer proceeds through participation of neighbouring group i.e. iodo group while such neighbouring group participation by iodo group is not possible in the cis-isomer. Hence, cis-isomer undergoes the normal $S{{N}^{2}}$ mechanism. Mechanism can be shown as follows:

Hence we can say that option A is the correct answer.
Note:
$S{{N}^{2}}$ is generally a type of nucleophilic substitution reaction. It is one step reaction in which one bond is broken and one bond is formed synchronously. If the substrate under nucleophilic attack is chiral in nature then this often leads to inversion of configuration which is called as a Walden inversion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




