
$3p_y$ orbital has which nodal plane?
A) XY
B) YZ
C) ZX
D) All of these.
Answer
558.6k+ views
Hint:First of all we should be aware of nodal planes. Hence, a nodal plane is a plane in which the probability of finding an electron is almost zero. The coordinates of these planes are obtained by solving the equation of Schrödinger wave for atoms or molecules such that to find the shape of atomic and molecular orbitals.
Complete step-by-step answer:There are only two nodes in a given orbital and these are Radial Node and the Angular Node.
The spherical surface where the probability of obtaining an electron is zero is termed as the Radial Node. Radial node is also known as the nodal region.
The term Angular node is also known as the nodal plane, the plane that passes through the nucleus. It is generally equal to the azimuthal quantum number (l).
Only the 2s orbital consists of a nodal shell, whereas the 2pz or the 2p orbitals have a nodal plane.
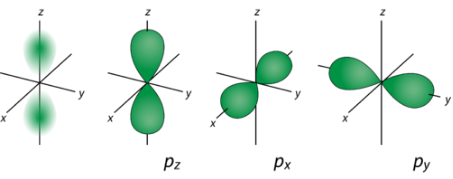
The formula for finding the total number of nodes is as follows.
Total no. of nodes = (n – l - 1).
Here, (n-1) is the total no. of nodes.
The option C has only value of (l=1).
Thus, $3p_y$ has ZX as the nodal plane.
Hence, the option C) is correct.
Note:The nodal surface is also known as a radial node. It is just a hollow spherical region where no electrons are found. There are three nodal planes found in 3p subshell, one from each (3px, 3py, 3pz).
Complete step-by-step answer:There are only two nodes in a given orbital and these are Radial Node and the Angular Node.
The spherical surface where the probability of obtaining an electron is zero is termed as the Radial Node. Radial node is also known as the nodal region.
The term Angular node is also known as the nodal plane, the plane that passes through the nucleus. It is generally equal to the azimuthal quantum number (l).
Only the 2s orbital consists of a nodal shell, whereas the 2pz or the 2p orbitals have a nodal plane.

The formula for finding the total number of nodes is as follows.
Total no. of nodes = (n – l - 1).
Here, (n-1) is the total no. of nodes.
The option C has only value of (l=1).
Thus, $3p_y$ has ZX as the nodal plane.
Hence, the option C) is correct.
Note:The nodal surface is also known as a radial node. It is just a hollow spherical region where no electrons are found. There are three nodal planes found in 3p subshell, one from each (3px, 3py, 3pz).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




