
4-methyl benzene sulphonic acid reacts with sodium acetate to give:
Answer
560.7k+ views
Hint: 4-methyl benzene sulphonic acid is aromatic sulphonic acid. It is considered as a derivative of sulphuric acid. The hydrogen atom of sulfonic acid group is highly acidic. The hydrogen atom of sulfonic acid group reacts with sodium acetate and results in the formation of salt.
Complete step-by-step answer:
We have to determine the product of the following reaction.
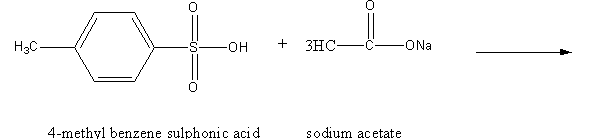
The hydrogen atom of the sulfonic acid group is highly acidic due to the following reasons.
As we know that oxygen atom is more electronegative than the sulphur atom. Due to the presence of electronegative oxygen atoms the hydrogen atom of the sulfonic acid group can be released easily.
Also, the sulfonate ion is stabilized due to the dispersal of negative charge over three oxygen atoms.
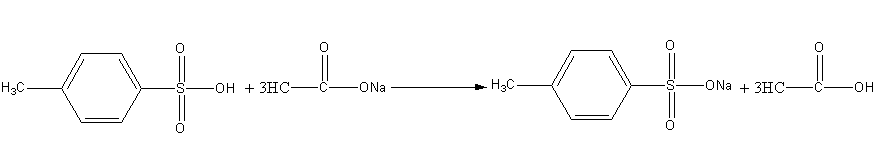
Sodium acetate is basic in nature so the reaction of 4-methyl benzene sulphonic acid with sodium acetate is an acid-base reaction. Here, acetate ion abstracts the acidic proton of 4-methyl benzene sulphonic acid and gives acetic acid and sodium salt of 4-methyl benzene sulphonate as the product.
Here is the salt formation reaction.
Note: Aromatic sulfonic acids are prepared by reaction of an aromatic compound with sulphuric acids. The reaction is known as sulphonation reaction. In this reaction hydrogen atom of the benzene, the group is replaced by the sulfonic acid group. Depending on the reagent used aromatic sulfonic acids show various reactions like reactions involving hydrogen atoms of sulfonic acid group, a reaction involving hydroxyl group of sulfonic acid group and reaction involving the whole of sulfonic acid group.
Complete step-by-step answer:
We have to determine the product of the following reaction.

The hydrogen atom of the sulfonic acid group is highly acidic due to the following reasons.
As we know that oxygen atom is more electronegative than the sulphur atom. Due to the presence of electronegative oxygen atoms the hydrogen atom of the sulfonic acid group can be released easily.
Also, the sulfonate ion is stabilized due to the dispersal of negative charge over three oxygen atoms.
Sodium acetate is basic in nature so the reaction of 4-methyl benzene sulphonic acid with sodium acetate is an acid-base reaction. Here, acetate ion abstracts the acidic proton of 4-methyl benzene sulphonic acid and gives acetic acid and sodium salt of 4-methyl benzene sulphonate as the product.
Here is the salt formation reaction.

Note: Aromatic sulfonic acids are prepared by reaction of an aromatic compound with sulphuric acids. The reaction is known as sulphonation reaction. In this reaction hydrogen atom of the benzene, the group is replaced by the sulfonic acid group. Depending on the reagent used aromatic sulfonic acids show various reactions like reactions involving hydrogen atoms of sulfonic acid group, a reaction involving hydroxyl group of sulfonic acid group and reaction involving the whole of sulfonic acid group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




