
What would a Bohr model for magnesium look like?
Answer
535.8k+ views
Hint: Draw a nucleus with required number of shells. Since the total number of electrons in a magnesium atom are 12. So start filling them up in the shells around the nucleus one by one keeping in mind the number of electrons a shell can accommodate.
Complete answer:
Magnesium (chemical symbol: Mg) is a s block element and has atomic number 12. It is an extremely important mineral as it is involved in various reactions taking place in our body. Its electronic configuration can be written as follows:-
$[Ne]3{{s}^{2}}$
-Neil Bohr proposed an early model of an atom in which central nuclei containing neutrons and protons are surrounded by electrons revolving around them in different shells. In all electrically neutral atoms, the no. of electrons = no. of protons which are certainly equal to the atomic number of the element which makes it different from every other element.
-Electron filling is done in a consistent manner which is instructed as follows:-
Under the standard conditions, we must start from the innermost shell and fill only 2 electrons in it and move to next.
Then according to octet rule, the next shell must not accommodate more than 8 electrons and so on.
We can now begin with filling of 12 electrons around a nucleus containing 12 protons and 12 neutrons.
-Make a nucleus with 12 protons and 12 neutrons. Now fill 2 electrons in the innermost shell (K-shell) and then 8 electrons in the next shell (L-shell). Since we had 12 electrons among which 10 are already accommodated so last 2 electrons can be fitted in the next shell at the end.
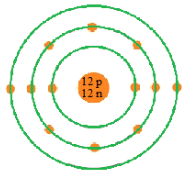
This is the Bohr model of magnesium with 12 electrons revolving around it in different shells (energy levels).
Note: The electrons in the outermost shell or energy level are helpful in finding the properties of that particular element. Also these electrons are known as valence electrons which play a vital role in reactions. The total no. of valence electron of magnesium = 2
Complete answer:
Magnesium (chemical symbol: Mg) is a s block element and has atomic number 12. It is an extremely important mineral as it is involved in various reactions taking place in our body. Its electronic configuration can be written as follows:-
$[Ne]3{{s}^{2}}$
-Neil Bohr proposed an early model of an atom in which central nuclei containing neutrons and protons are surrounded by electrons revolving around them in different shells. In all electrically neutral atoms, the no. of electrons = no. of protons which are certainly equal to the atomic number of the element which makes it different from every other element.
-Electron filling is done in a consistent manner which is instructed as follows:-
Under the standard conditions, we must start from the innermost shell and fill only 2 electrons in it and move to next.
Then according to octet rule, the next shell must not accommodate more than 8 electrons and so on.
We can now begin with filling of 12 electrons around a nucleus containing 12 protons and 12 neutrons.
-Make a nucleus with 12 protons and 12 neutrons. Now fill 2 electrons in the innermost shell (K-shell) and then 8 electrons in the next shell (L-shell). Since we had 12 electrons among which 10 are already accommodated so last 2 electrons can be fitted in the next shell at the end.

This is the Bohr model of magnesium with 12 electrons revolving around it in different shells (energy levels).
Note: The electrons in the outermost shell or energy level are helpful in finding the properties of that particular element. Also these electrons are known as valence electrons which play a vital role in reactions. The total no. of valence electron of magnesium = 2
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




